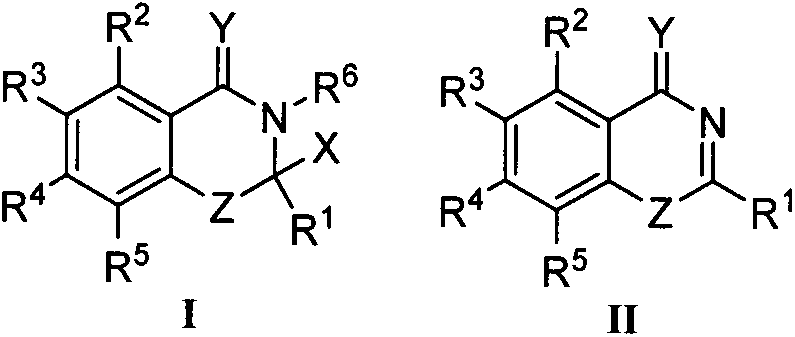
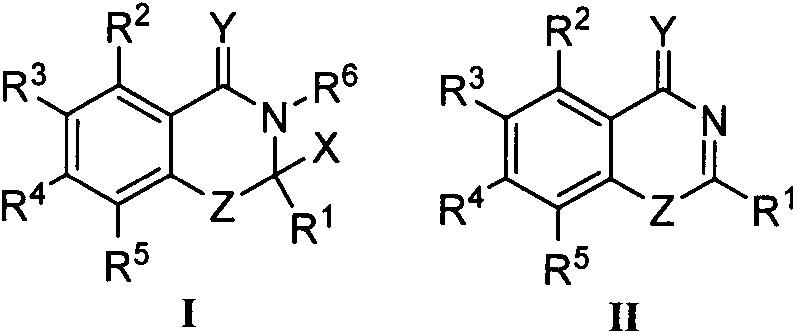
Benzoxazinone derivative and preparation method and application thereof
A technology of benzoxazinone and its derivatives, which is applied in the field of medicinal chemistry, and can solve the problems of inability to realize mass production preparation, limited application, and no economical and efficient synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of some compounds of the present invention, including Ia-Ih, taking compound Ia as an example
[0029] Preparation of N-benzyl-2-hydroxy-5-methoxybenzamide:
[0030]
[0031] Dissolve 1 g (5.95 mmol) of 5-methoxysalicylic acid in 30 ml of anhydrous dichloromethane, add 2.14 g (10.7 mmol) of thionyl chloride, 1 drop of N,N-dimethylformamide, After heating up to reflux for 2 hours, cool to room temperature, spin dry to remove solvent, dissolve with anhydrous dichloromethane, add dropwise to anhydrous diethylamine containing 1.85g (17.80mmol) of triethylamine and 0.76g (7.14mmol) of benzylamine. in methyl chloride solution. After the dropwise addition was completed, the mixture was stirred at room temperature for 5 min, and spin-dried to obtain a crude product, 1.27 g of white solid N-benzyl-2-hydroxy-5-methoxybenzamide purified by column chromatography, with a yield of 83%. 1 H NMR (300MHz, DMSO) δ12.62(s, 1H), 7.36-7.32(m, 5H), 7.22(d, J=9.3Hz, 1...
Embodiment 2
[0056] Example 2 Experiment of antitumor activity of compounds Ia-Ih.
[0057] Experimental Materials
[0058] Cell line: human cervical cancer cell HeLa and human breast cancer cell MCF-7; sample: compound Ia-Ih was added with appropriate amount of DMSO to make a concentration of 10 -2 M solution, diluted with culture medium to the corresponding concentration before use.
[0059] experimental method
[0060] Cell digestion and counting, 2.5×10 per well 3 Cells were placed in a 96-well plate with a total volume of 100 μl per well and cultured for 24 hours; the samples to be tested were diluted to an appropriate concentration with medium, 100 μl per well, and three replicate wells were set. After culturing for 72 hours, add 20 μl of 5 mg / ml MTT solution, after incubation for 4 hours, suck out all the liquid in the well, add 100 μl of DMSO, and measure the absorbance with a full-wavelength microplate reader; inhibition rate=(1-average absorbance value of the administration gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com