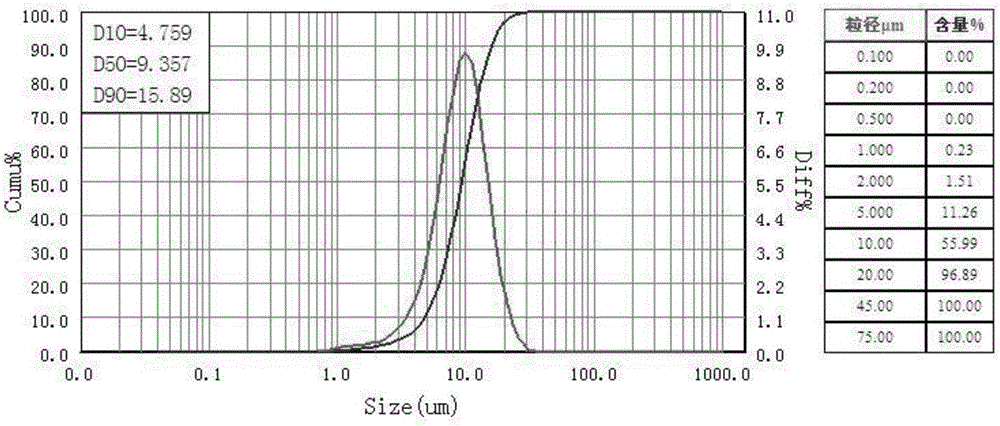
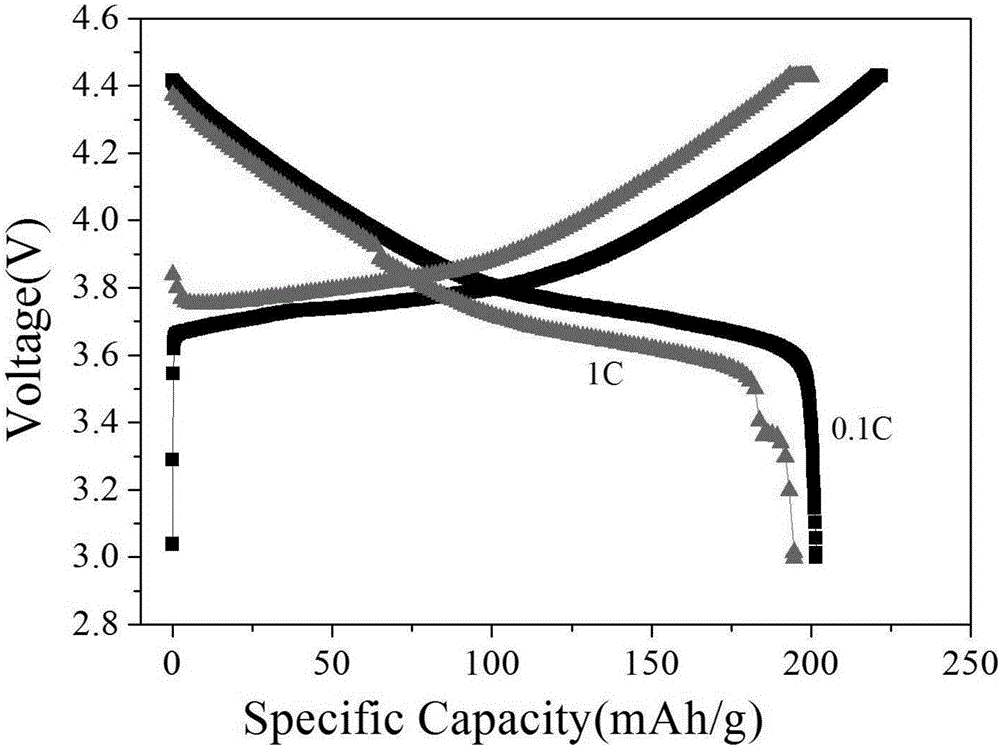
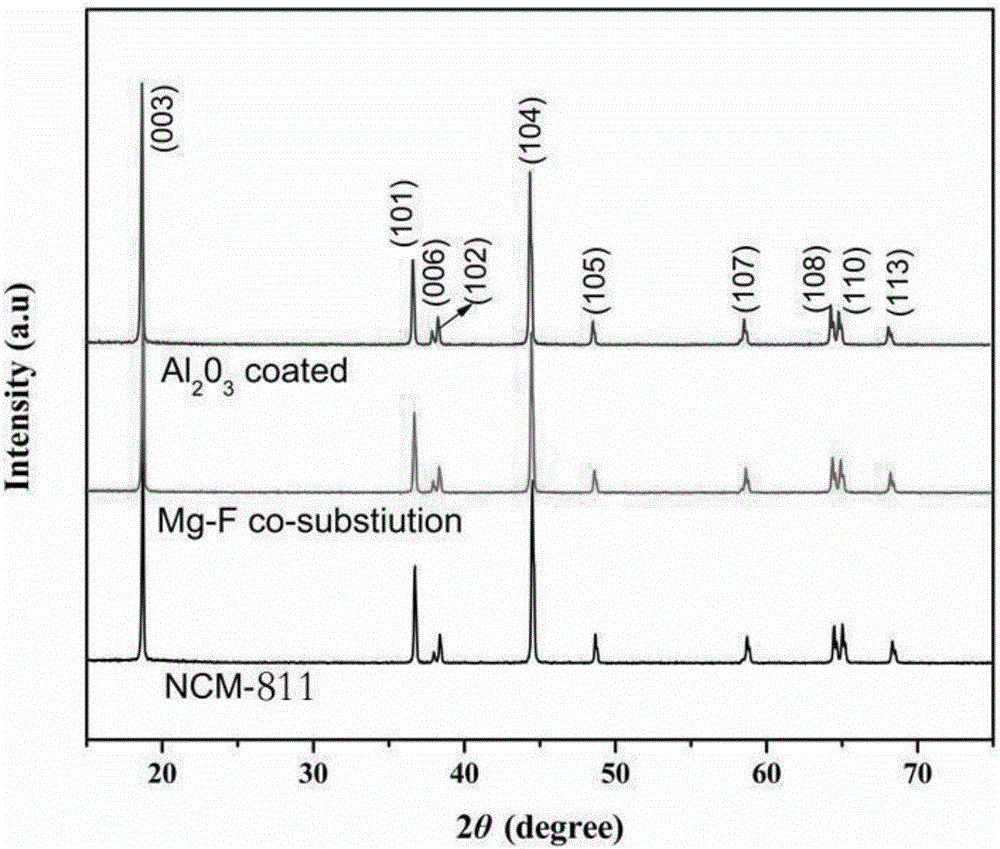
Lithium NCM (nickel cobalt manganese) material and preparation method thereof
A technology of nickel-cobalt lithium manganese oxide and positive electrode materials, which is applied in the field of nickel-cobalt lithium manganese oxide materials and its preparation, can solve the problems of poor cycle performance and thermal stability, and reduced material processing performance, so as to improve the degree of mixing, Effects of inhibiting corrosion, improving structural stability and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of composite anion-cation co-doped LiNi 0.78 Mg 0.01 al 0.01 co 0.1 mn 0.1 o 1.98 f 0.02 Cathode material
[0029] Weigh NiSO according to Ni: Co: Mn = molar ratio of 0.8:0.1:0.1 4 、CoSO 4 , MnSO 4 , according to the ratio of 1mol / L, configure it into a salt solution, add it to the reaction kettle, control the solution temperature at 50-60°C, and feed N 2Protect the atmosphere, control the rotation speed of the stirred tank, slowly add 1mol / L complexing agent ammonia water, and then add 0.5mol / L precipitant Na 2 CO 3 solution, the process kept the pH of the solution at 11.5, then stirred and formed for 4 hours to complete the coprecipitation reaction. After the fully reacted slurry is washed with deionized water for 3 times, it has been configured into a 0.1mol / L Mg-Al salt or Mg-Ti mixed solution according to M:A:B=1:0.01:0.01, and the solid content is controlled at 40%, stirring and mixing for 2 hours, and drying the slurry by atomization dryin...
Embodiment 2
[0030] Example 2 Preparation of composite anion and cation co-doped LiNi 0.77 Mg 0.03 Ti 0.03 co 0.1 mn 0.1 o 1.97 f 0.03 Cathode material
[0031] Weigh NiSO according to Ni: Co: Mn = molar ratio of 0.8:0.1:0.1 4 、CoSO 4 , MnSO 4 , according to the ratio of 1mol / L, configure it into a salt solution, add it to the reaction kettle, control the solution temperature at 50-60°C, and feed N 2 Protect the atmosphere, control the rotation speed of the stirred tank, slowly add 1mol / L complexing agent ammonia water, and then add 0.5mol / L precipitant Na 2 CO 3 solution, the process kept the pH of the solution at 11.5, then stirred and formed for 4 hours to complete the coprecipitation reaction. After the fully reacted slurry was washed with deionized water for 3 times, according to M:A:B=1:0.03:0.03, it has been configured into a 0.1mol / L Mg-Ti salt mixed solution, and the solid content is controlled at 40%. Stir After mixing for 2 hours, the slurry was dried by atomization ...
Embodiment 3
[0032] Example 3 Preparation of composite anion and cation co-doped LiNi 0.795 Mg 0.005 Al 0.005 co 0.1 mn 0.1 o 1.995 f 0.005 Cathode material
[0033] Weigh NiSO according to Ni: Co: Mn = molar ratio of 0.8:0.1:0.1 4 、CoSO 4 , MnSO 4 , according to the ratio of 1mol / L, configure it into a salt solution, add it to the reaction kettle, control the solution temperature at 50-60°C, and feed N 2 Protect the atmosphere, control the rotation speed of the stirred tank, slowly add 1mol / L complexing agent ammonia water, and then add 0.5mol / L precipitant Na 2 CO 3 solution, the process kept the pH of the solution at 11.5, then stirred and formed for 4 hours to complete the coprecipitation reaction. After the fully reacted slurry is washed with deionized water for 3 times, it has been configured into a 0.1mol / L Mg-Ti salt mixed solution according to M:A:B=1:0.005:0.005, and the solid content is controlled at 40%. Stir After mixing for 2 hours, the slurry was dried by atomiza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com