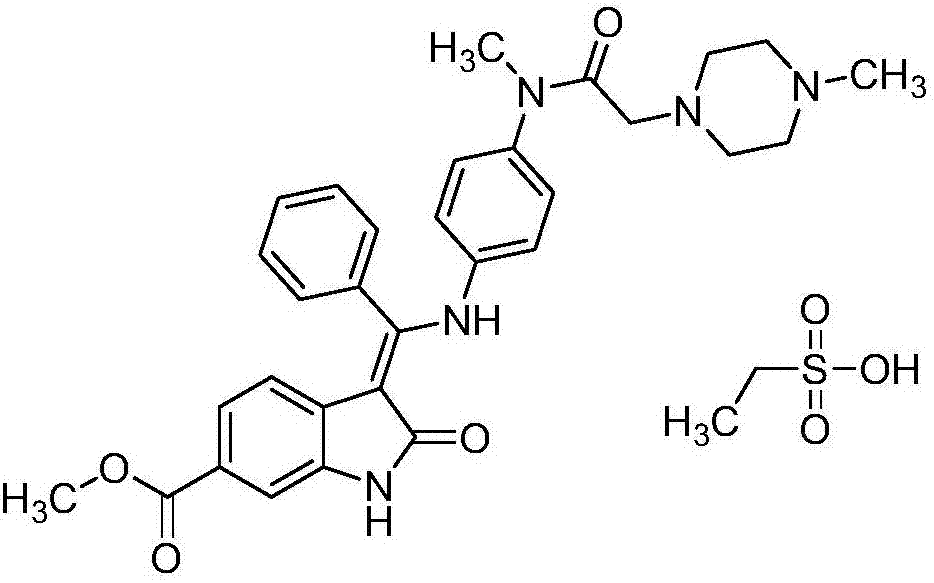
Nintedanib self-microemulsion preparation and soft capsule thereof and preparation method
A technology for nintedanib and emulsion preparations, which is applied in the directions of capsule delivery, pharmaceutical formulation, emulsion delivery, etc., can solve the problem of difficulty in improving the bioavailability of such drugs, so as to improve the bioavailability, speed and degree of , the effect of obvious social benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
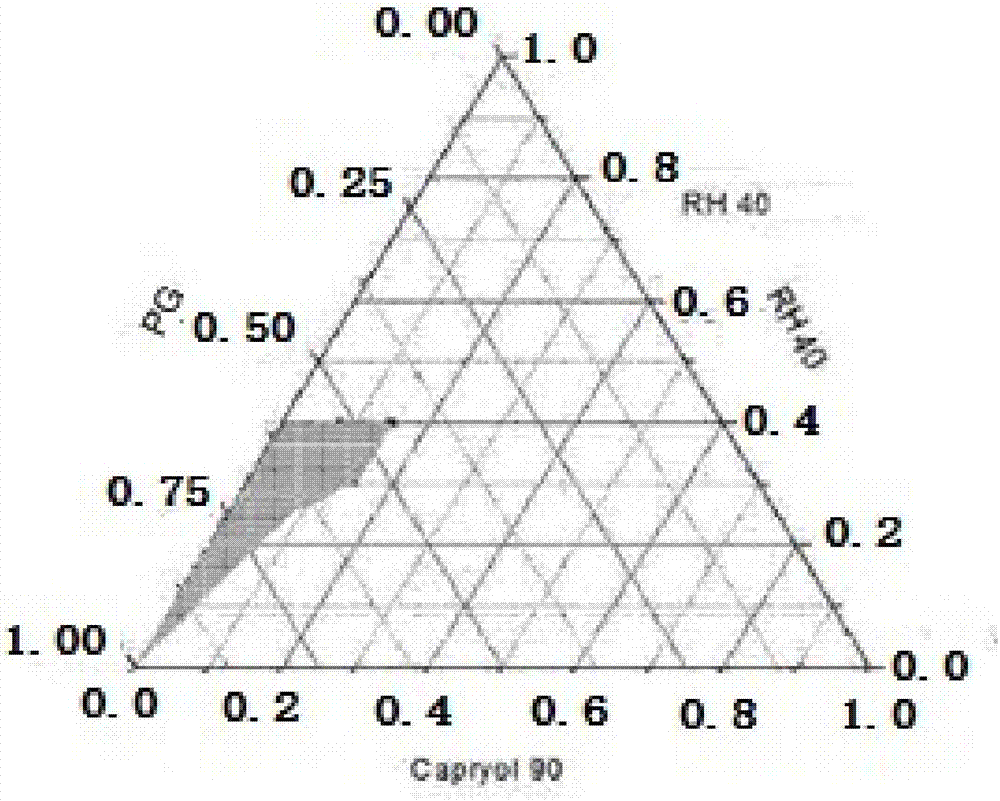
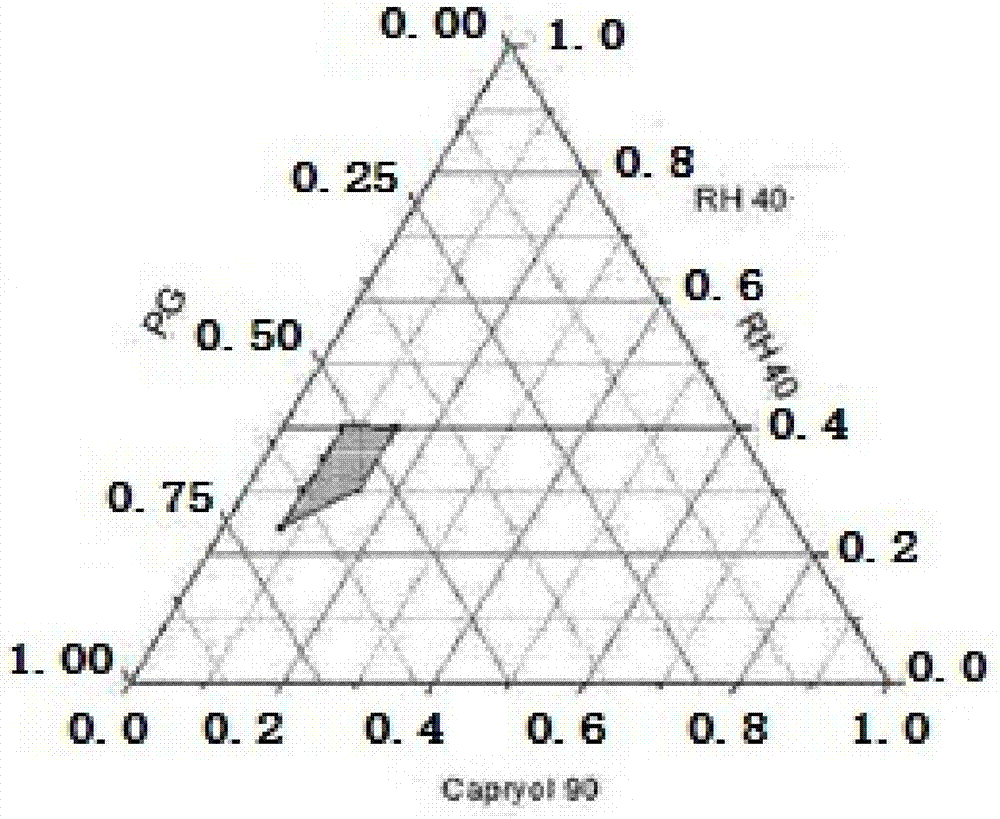
[0033] Propylene glycol monocaprylate (Capryol 90) is used as the oil phase, polyoxyethylene 40 hydrogenated castor oil (RH40) is used as the emulsifier, and propylene glycol (PG) is used as the co-emulsifier. Mix the emulsifier and co-emulsifier evenly in the weight ratio of 2:1, 1:1, 1:2, and then mix it with the oil phase in the weight ratio of 1:9, 2:8, 3:7, 4:6, 5:5 , 6:4, 7:3, 8:2, 9:1 mixed at 50r·min -1 Drop into water preheated to 37°C while stirring, and observe the state of microemulsion. Record the changes and states of the mixing system in different proportions, and limit the proportion of emulsifier to 20%-40%, the proportion of co-emulsifier to more than 20%-60%, and the proportion of oil phase to 10%-30%. These proportioning data draw the ternary phase diagram, and select the effective area of the blank self-microemulsion and the effective area of nintedanib self-microemulsion, see figure 1 Middle shaded part.
[0034] From figure 2 It can be seen tha...
Embodiment 2
[0037] Prescription of nintedanib self-microemulsion preparation with nintedanib concentration of 2%
[0038] Select raw materials according to the following proportions:
[0039]
[0040] Preparation method: Dissolve the prescribed amount of nintedanib in the prescribed amount of PG, add the prescribed amount of MCT and RH40, mix well, and obtain nintedanib self-microemulsion preparation.
[0041]The prepared nintedanib self-microemulsion preparation is a colorless uniform transparent liquid. Take the prepared nintedanib self-microemulsion preparation, dilute 100 times with water, measure the particle size, and the average particle size is 18.3nm. Take nintedanib self-microemulsion 5ml, at 50r min -1 Add 10 times the amount of water at 37°C under stirring, and the self-microemulsion stock solution can be completely emulsified within 1 minute, forming a transparent O / W type microemulsion with slightly bluish light, and at 50r·min -1 It remained stable for 8 hours under s...
Embodiment 3
[0043] Prescription of nintedanib self-microemulsion preparation with nintedanib concentration of 2.5%
[0044] Select raw materials according to the following proportions:
[0045]
[0046] Preparation method: Dissolve the prescribed amount of nintedanib in the prescribed amount of PG, add the prescribed amount of MCT and RH40, mix well, and obtain nintedanib self-microemulsion preparation.
[0047] The prepared nintedanib self-microemulsion preparation is a colorless uniform transparent liquid. Take the prepared nintedanib self-microemulsion preparation, dilute 100 times with water, measure the particle size, and the average particle size is 18.1nm. Take nintedanib self-microemulsion 5ml, at 50r min -1 Add 10 times the amount of water at 37°C under stirring, and the self-microemulsion stock solution can be completely emulsified within 1 minute, forming a transparent O / W type microemulsion with slightly bluish light, and at 50r·min -1 It remained stable for 8 hours unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com