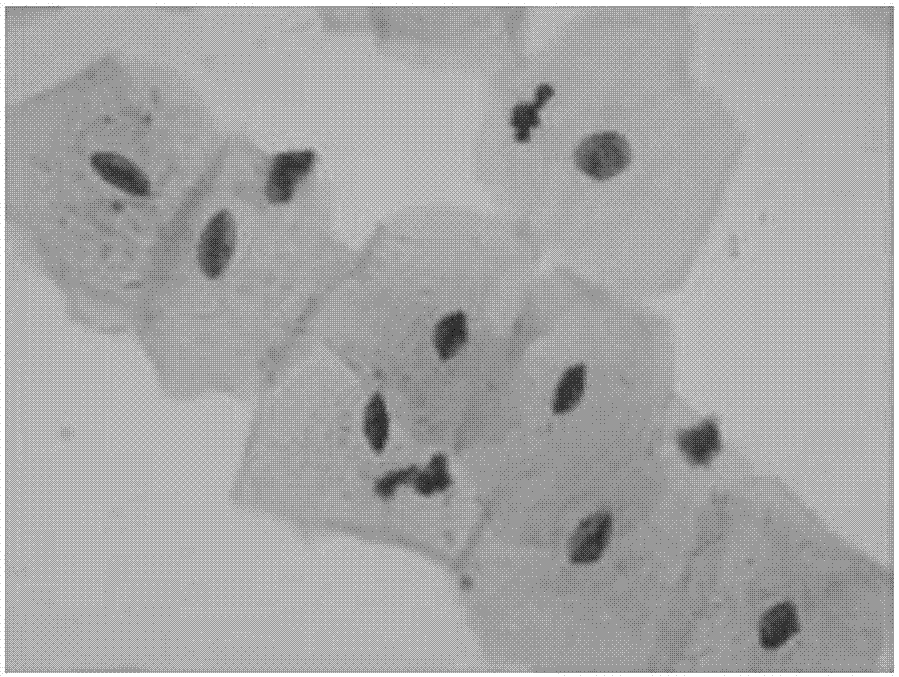
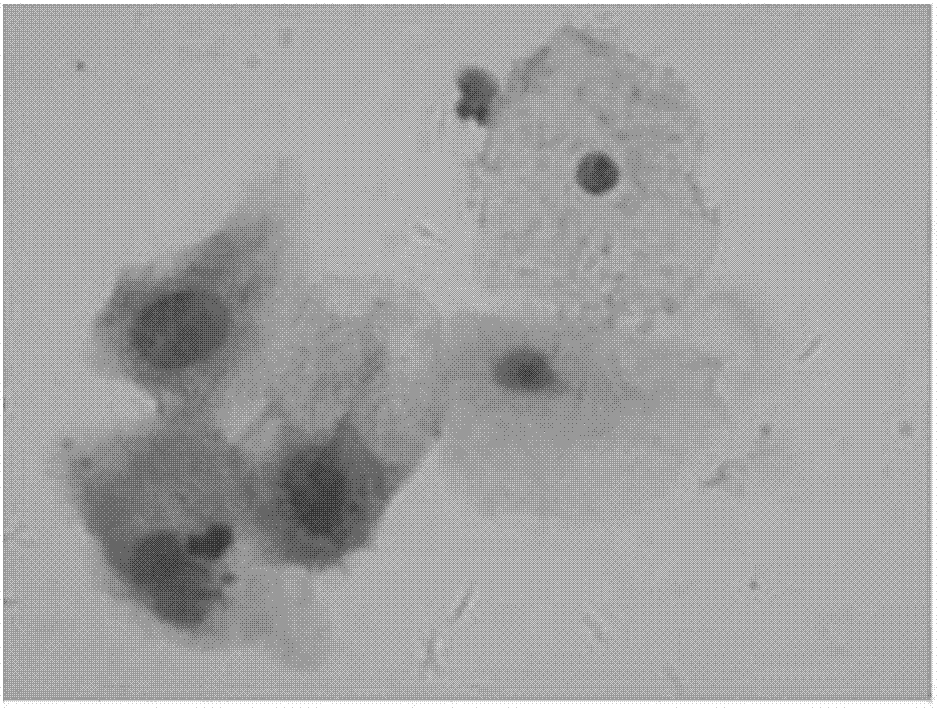
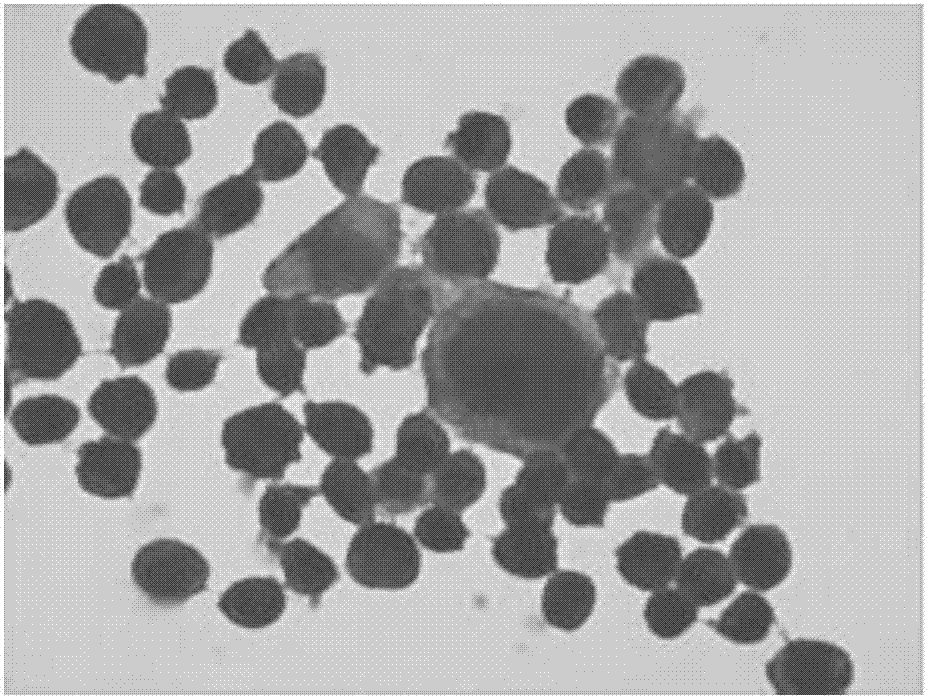
P16INK4a immunolabeling color-developing kit for cervical liquid-based cells
A p16ink4a, liquid-based cell technology, applied in the field of clinical medical pathology, can solve problems such as limitations, non-specificity of immunostaining, false negative staining results, etc., and achieve the effects of avoiding the influence of antigens, strong specificity, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 A method for cervical liquid-based cell p16 INK4a Immunolabeling Chromogenic Kit
[0049] The kit includes: p16 INK4a Antigen Preservation Solution, Primary Antibody, Secondary Antibody, DAB Chromogenic Solution, Endogenous Peroxidase Blocker, Blocking Solution, Buffer, Positive Control and Negative Control.
[0050] Among them, the p16 INK4a The antigen preservation solution is composed of the following components and their concentrations: NaCl 85mmol / L, KCl7.5mmol / L, edetate disodium 3.5mmol / L, urea 42mmol / L, sodium citrate 3.6mmol / L, sucrose 16mmol / L L, PEG-400 6.5mmol / L, paraformaldehyde 2%, glycerin 8.4mmol / L and dithiothreitol 16.5mmol / L.
[0051] The primary antibody is p16 INK4a mouse anti-human monoclonal antibody; the second antibody is a rabbit anti-mouse IgG antibody labeled with horseradish peroxidase.
[0052] The endogenous peroxidase blocking agent is 0.75% periodic acid solution; the blocking solution is animal non-immune goat serum.
[...
Embodiment 2
[0055] Example 2 A method for cervical liquid-based cell p16 INK4a Immunolabeling Chromogenic Kit
[0056] A liquid-based cell p16 for cervix INK4a Immunolabeling chromogenic kit, the kit includes: p16 INK4a Antigen Preservation Solution, Primary Antibody, Secondary Antibody, DAB Chromogenic Solution, Endogenous Peroxidase Blocker, Blocking Solution, Buffer, Positive Control and Negative Control.
[0057] The p16 INK4a The antigen preservation solution is composed of the following components and their concentrations: NaCl 60mmol / L, KCl 2mmol / L, disodium edetate 1mmol / L, urea 30mmol / L, sodium citrate 1mmol / L, sucrose 5mmol / L, PEG -4004mmol / L, paraformaldehyde 2%, glycerol 2mmol / L and dithiothreitol 10mmol / L.
[0058] Wherein, the first antibody is p16 INK4a mouse anti-human monoclonal antibody; the second antibody is a rabbit anti-mouse IgG antibody labeled with horseradish peroxidase.
[0059] The endogenous peroxidase blocking agent is 0.5% periodic acid solution; the b...
Embodiment 3
[0062] Example 3 A method for cervical liquid-based cell p16 INK4a Immunolabeling Chromogenic Kit
[0063] A liquid-based cell p16 for cervix INK4a Immunolabeling chromogenic kit, the kit includes: p16 INK4a Antigen Preservation Solution, Primary Antibody, Secondary Antibody, DAB Chromogenic Solution, Endogenous Peroxidase Blocker, Blocking Solution, Buffer, Positive Control and Negative Control.
[0064] Among them, the p16 INK4a The antigen preservation solution is composed of the following components and their concentrations: NaCl 100mmol / L, KCl10mmol / L, EDTA disodium 5mmol / L, urea 50mmol / L, sodium citrate 5mmol / L, sucrose 20mmol / L, PEG- 40010mmol / L, paraformaldehyde 2.5%, glycerol 10mmol / L and dithiothreitol 20mmol / L.
[0065] The first antibody is p16 INK4a mouse anti-human monoclonal antibody; the second antibody is a rabbit anti-mouse IgG antibody labeled with horseradish peroxidase.
[0066] The endogenous peroxidase blocking agent is 1% periodic acid solution; t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com