Synthesis technology ofminoxidil
A synthesis process and reaction kettle technology, applied in the field of pharmaceutical synthesis, can solve problems such as low yield and complexity, and achieve the effects of mature production process, low pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
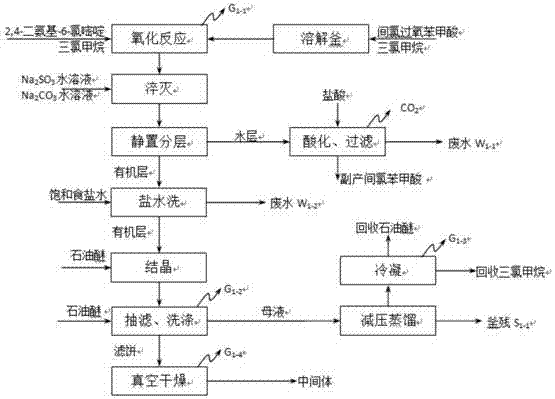
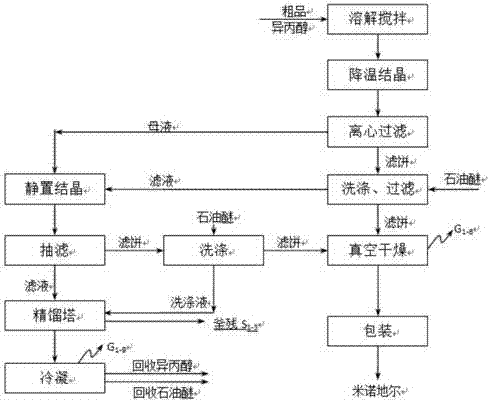
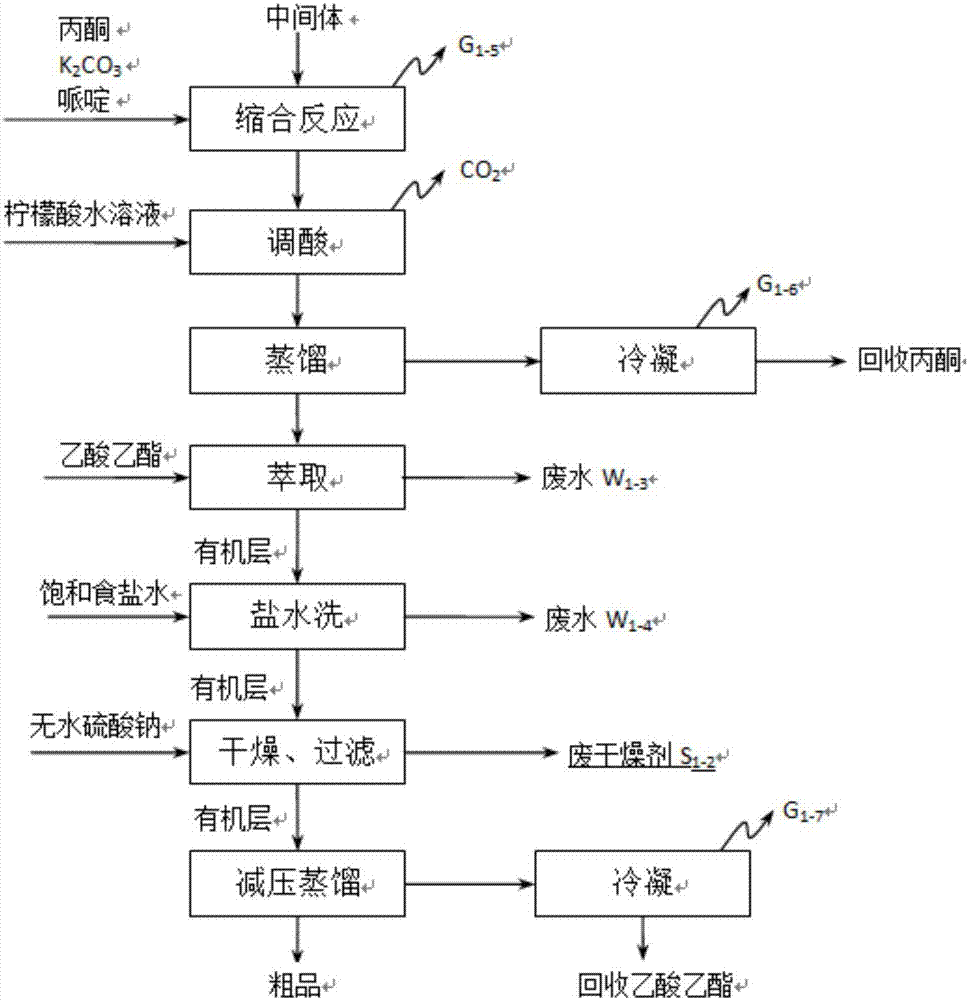
[0048] A kind of synthetic technique of minoxidil, comprises the following steps:
[0049] The first step: oxidation reaction generates intermediate 2,6-diamino-4-chloropyrimidine-1-oxide,
[0050] One: Reaction Equation
[0051]
[0052] Two: material ratio table
[0053]
[0054]
[0055] Three: specific operation
[0056] 1. Add 310kg of chloroform into a clean 2T reaction kettle, and the temperature inside the kettle is 17°C;
[0057] 2. Slowly add 150.0 kg of 2,4-diamino-6-chloropyrimidine solid, there is a slight exotherm when adding, and there is no need to use an ice-water bath to cool down;
[0058] 3. Heating to reflux;
[0059] 4. Add 620kg of chloroform and 189kg of m-chloroperoxybenzoic acid into another clean 2T reaction kettle to release heat without using an ice-water bath to cool down;
[0060] 5. Pump the chloroform solution of m-chloroperoxybenzoic acid into the high-level tank, and add it dropwise to the chloroform solution of 2,4-diamino-6-ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com