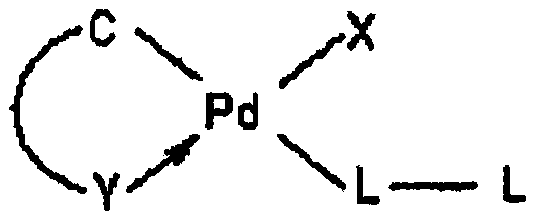
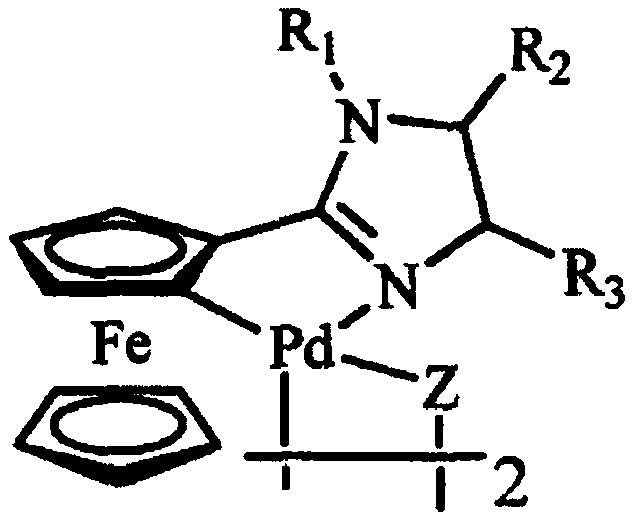
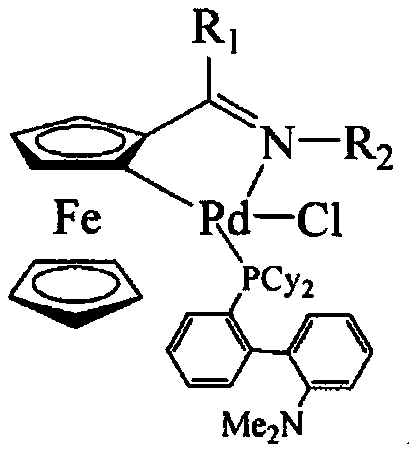
Aromatic heterocyclic alcohol ring palladium metal catalyst and its application
A metal catalyst and an aromatic heterocycle technology are applied in the synthesis and application of cyclic palladium metal catalysts, and in the field of aromatic heterocyclic alcohol ring palladium metal catalysts, which can solve the problems of poor universality and complex synthesis routes, and achieve low dosage and good application. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The following are specific examples of the present invention, which are only used to explain the present invention and not to limit.
[0047] Be as follows the synthetic method of aromatic heterocyclic alcohol ring palladium metal catalyst of the present invention:
[0048]
[0049] Specifically, the synthetic method of pyridine or quinoline heterocyclic alcohol compound typically includes:
[0050] Using 2,6-lutidine or 2-methylquinoline as raw material, anhydrous tetrahydrofuran (re-distilled with sodium) as solvent, in an anaerobic and anhydrous ultra-low temperature environment (-78°C), by controlling n-butyl The amount of base lithium is taken away from a proton on a methyl group, and then through nucleophilic addition to selected ketones or aldehydes or cyclic ether compounds, the corresponding heterocyclic alcohol compounds can be obtained in high yield.
[0051] The typical synthetic method of pyridine or quinoline heterocyclic alcohol cyclopalladium complex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com