Method for producing iron oxide yellow
A technology of iron oxide yellow and ferrous chloride, which is applied in the direction of iron oxide, iron oxide/iron hydroxide, etc., can solve the problem that the color consistency of iron oxide yellow is difficult to control, and achieve good color consistency, small Ca content, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: a kind of method of producing iron oxide yellow, comprises the steps:
[0019] S1: Add calcium carbonate and water into the reaction tank, the volume of the reaction tank is 50m 3 A stainless steel reactor, air is introduced from the bottom of the reactor and stirred continuously;
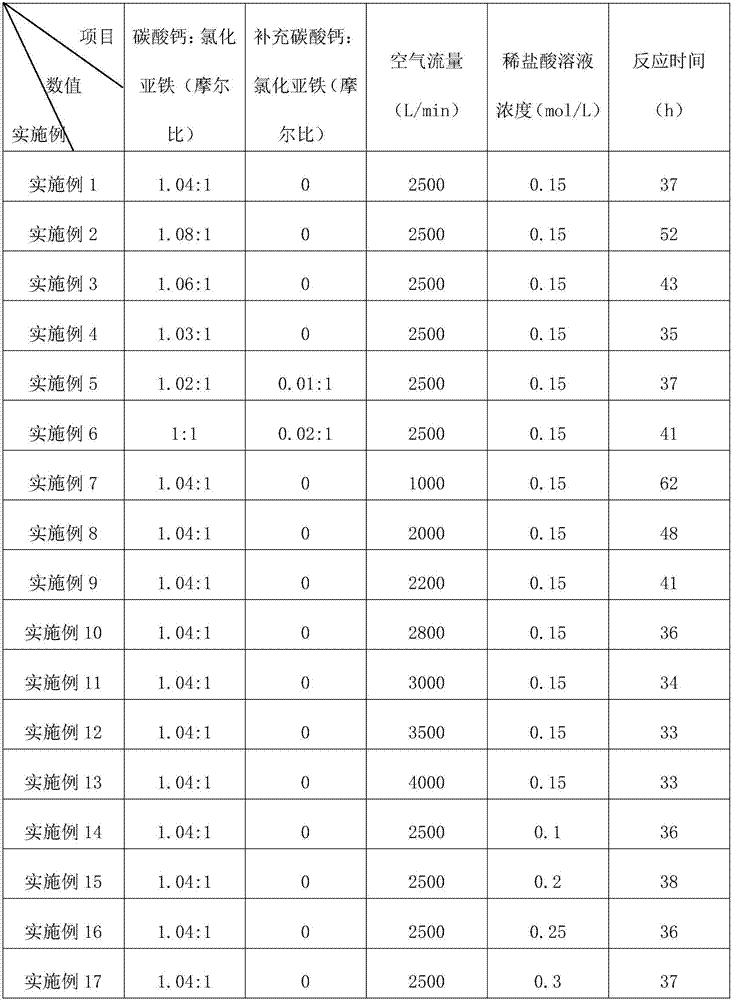
[0020] S2: Add ferrous chloride to the container, and the molar ratio of calcium carbonate and ferrous chloride is 1.04:1;
[0021] S3: Heat the system to 80°C, continuously blow in air during the process, and the air flow rate is 2500L / min; detect Fe in the system every 2h during the reaction process 2+ content, take 3 samples each time, and the time interval between each two samples is 5min; if Fe 2+ Content does not decline, then continue to react after supplementing calcium carbonate in the system, the mol ratio of the ferrous chloride that adds in the amount of calcium carbonate and step S2 is 0.01:1 at every turn; 2+ Content continues to decline, then continue to react...
Embodiment 2-17
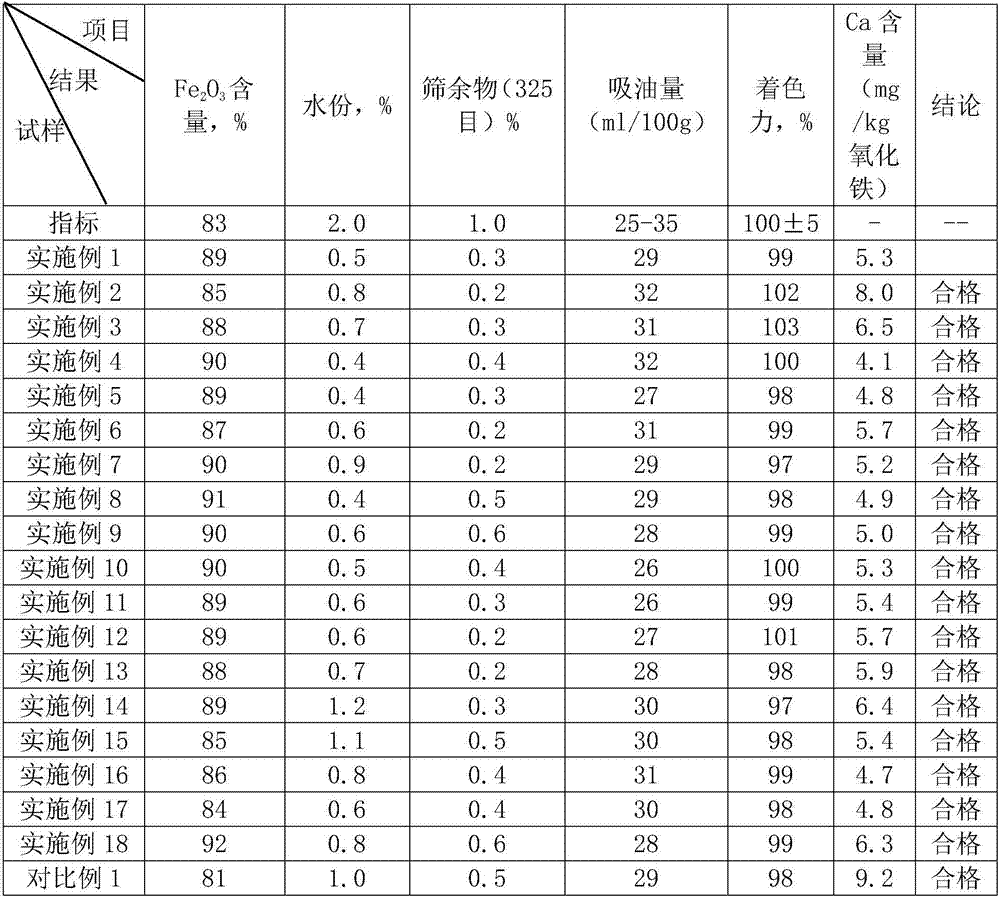
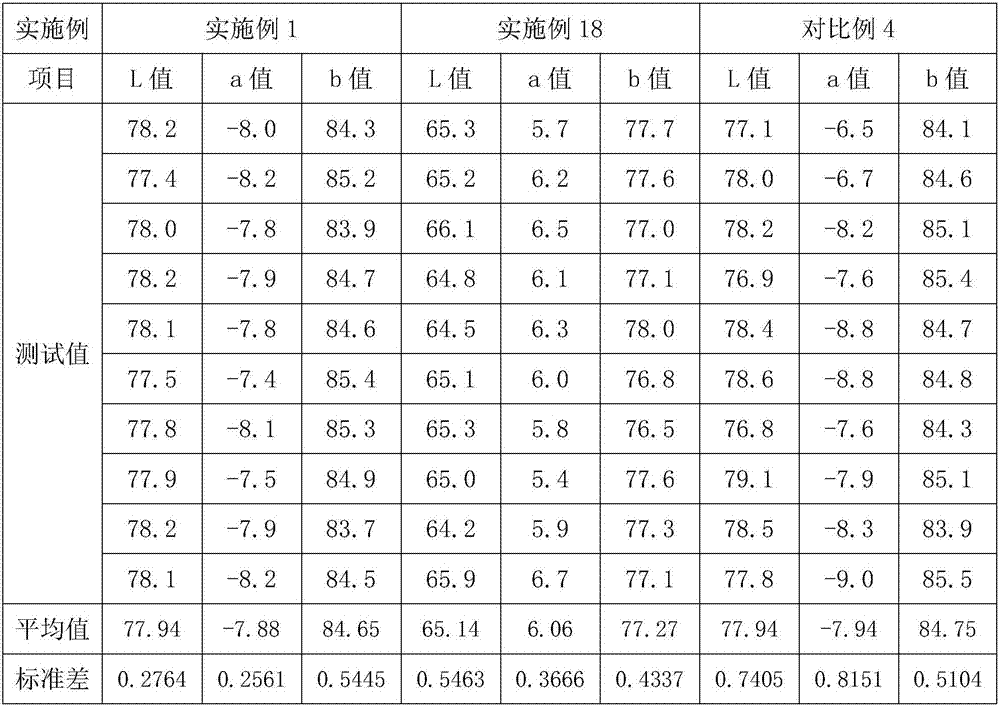
[0025] Embodiment 2-17: a kind of method of producing iron oxide yellow, and the difference of embodiment 1 is, the addition molar ratio of calcium carbonate and ferrous chloride, the molar ratio of supplementing calcium carbonate and ferrous chloride, reaction time, The air flow rate and the concentration of dilute hydrochloric acid solution are different, see Table 1 for specific parameters.
Embodiment 18
[0026] Embodiment 18: a kind of method of producing iron oxide yellow, comprises the steps:
[0027] S1: Add calcium carbonate and water into the reaction tank, the volume of the reaction tank is 50m 3 A stainless steel reactor, air is introduced from the bottom of the reactor and stirred continuously;
[0028] S2: Add ferrous chloride to the container, and the molar ratio of calcium carbonate and ferrous chloride is 1.04:1;
[0029] S3: Heat the system to 85°C, continuously blow in air during the process, and the air flow rate is 3000L / min; detect Fe in the system every 2h during the reaction process 2+ content, take 3 samples each time, and the time interval between each two samples is 5min; if Fe 2+ Content does not decline, then continue to react after supplementing calcium carbonate in the system, the mol ratio of the ferrous chloride that adds in the amount of calcium carbonate and step S2 is 0.01:1 at every turn; 2+ Content continues to decline, then continue to reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com