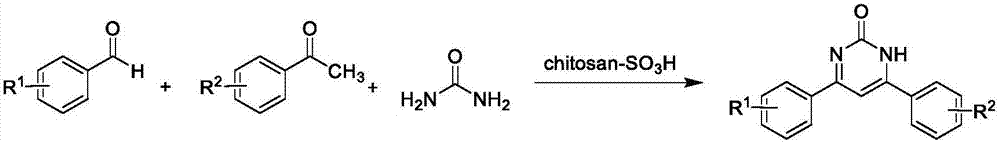
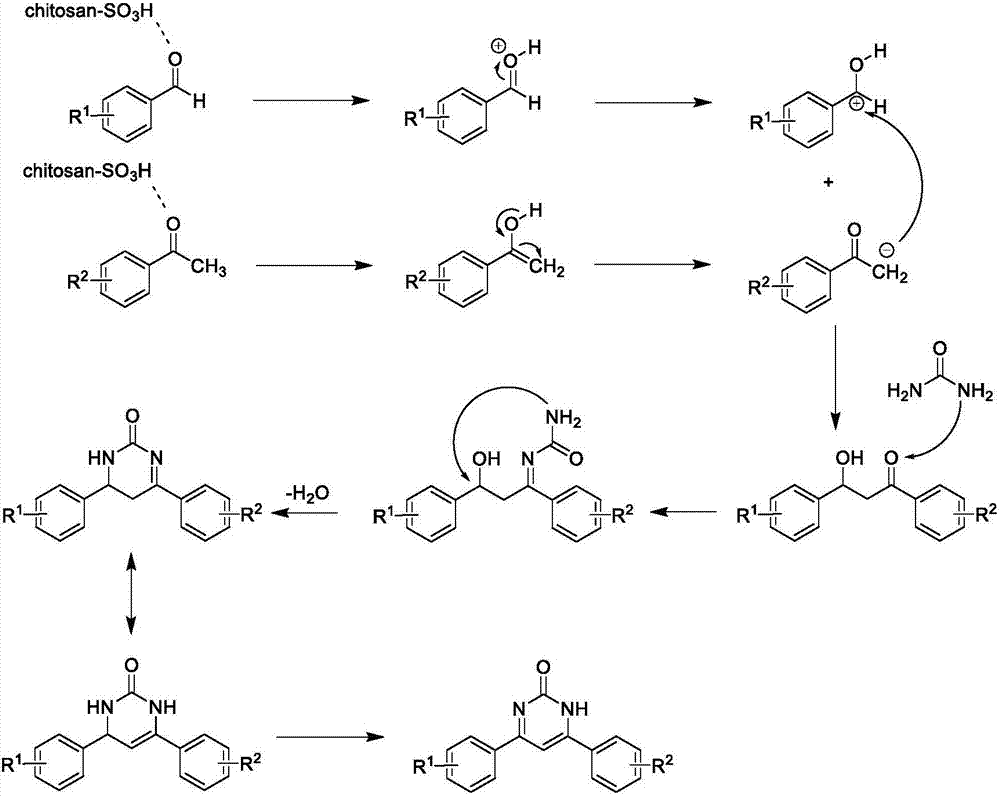
Method for catalytically synthesizing 4,6-diaryl pyrimidine-2(1H)-one derivative by using sulfonic acid functional chitosan
A technology for catalyzing chitosan and diarylpyrimidine is applied in the field of catalyzing the synthesis of 4,6-diarylpyrimidin-2-one compounds by sulfonic acid functionalized chitosan, and can solve the problems of low conversion rate and reaction temperature. high, long reaction time and other problems, to achieve the effect of easy separation and recycling, good biocompatibility, simple and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 0.11g (1.0mmol) benzaldehyde, 0.12g (1.0mmol) acetophenone, 0.07g (1.2mmol) urea and 15.4mg sulfonic acid functionalized chitosan catalyst were added in a single-necked flask, and were heated at 90 The reaction was heated at ℃, and the reaction was tracked and monitored by thin-layer chromatography; the reaction was completed after 30 minutes. After the reaction system was cooled to room temperature, ethyl acetate was added thereto, filtered, and the filtrate was distilled under reduced pressure. The obtained crude product was recrystallized with ethanol to obtain 4 , The pure product of 6-diphenylpyrimidin-2(1H)-one has a yield of 93%; the measured melting point is 216-218°C, which is consistent with the literature reports.
[0039] The filtered solid was washed three times with ethanol and dried in vacuum before being reused.
Embodiment 2
[0041] Add 0.12 g (1.0 mmol) 4-methylbenzaldehyde, 0.12 g (1.0 mmol) acetophenone, 0.07 g (1.2 mmol) urea, and 19.2 mg sulfonic acid functionalized chitosan catalyst into a one-necked flask without solvent Heating the reaction at 90°C under the conditions, using thin-layer chromatography to track and monitor the reaction, the reaction was completed after 35 minutes, after the reaction system was cooled to room temperature, ethyl acetate was added to it, filtered, the filtrate was distilled under reduced pressure, and the obtained crude product was recrystallized with ethanol The pure product of 4-(4-methylphenyl)-6-phenylpyrimidin-2(1H)-one can be obtained with a yield of 92%; the measured melting point is 288-289°C, which is consistent with the literature reports.
[0042] The filtered solid was washed three times with ethanol and dried in vacuum before being reused.
Embodiment 3
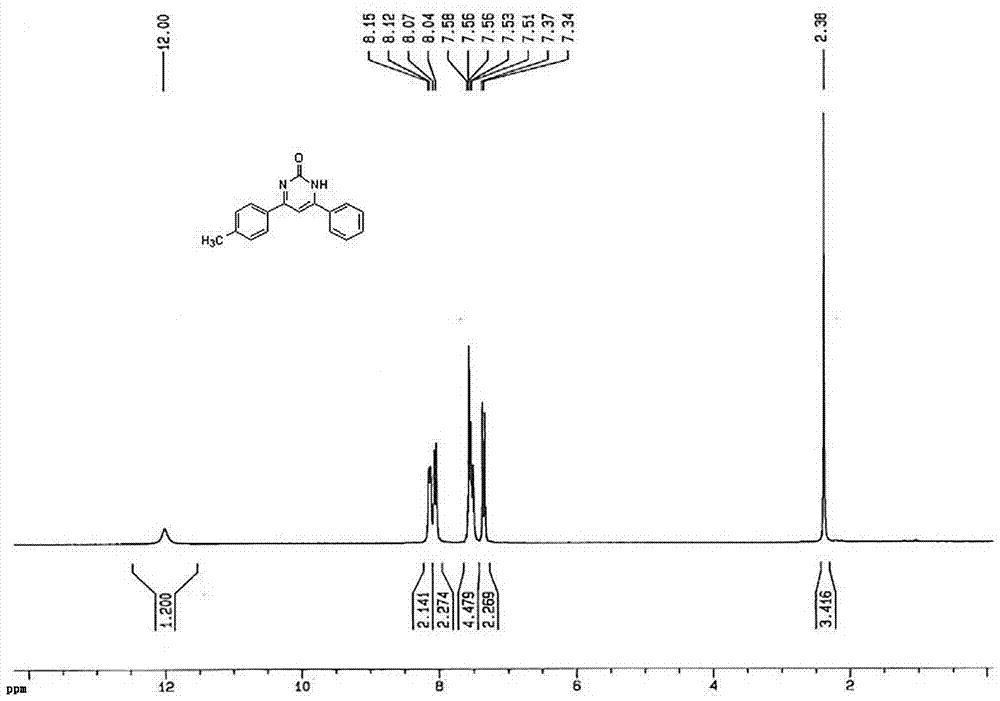
[0044] Add 0.11 g (1.0 mmol) benzaldehyde, 0.13 g (1.0 mmol) 4-methylacetophenone, 0.07 g (1.2 mmol) urea, and 19.8 mg sulfonic acid functionalized chitosan catalyst into a one-necked flask without solvent Heat the reaction at 90°C under the conditions, and use thin-layer chromatography to track and monitor the reaction; after 30 minutes, the reaction is completed. After the reaction system is cooled to room temperature, ethyl acetate is added to it, filtered, and the filtrate is distilled under reduced pressure. The obtained crude product is recrystallized with ethanol. The pure product of 4-phenyl-6-(4-methylphenyl)pyrimidin-2(1H)-one can be obtained with a yield of 90%; the measured melting point is 285-286° C., which is consistent with literature reports.
[0045] The filtered solid was washed three times with ethanol and dried in vacuum before being reused.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com