Composition for preparing hepatitis B vaccine and preparation method and application of composition
A hepatitis B vaccine and composition technology, which is applied in the directions of drug combinations, active ingredients of heterocyclic compounds, and medical preparations containing active ingredients, etc., can solve the problem that hepatitis B vaccine cannot prevent and clear chronic infection of hepatitis B virus at the same time, and achieves enhanced immunity. Effects of cytokine induction, strong immune activation effect, high antibody production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
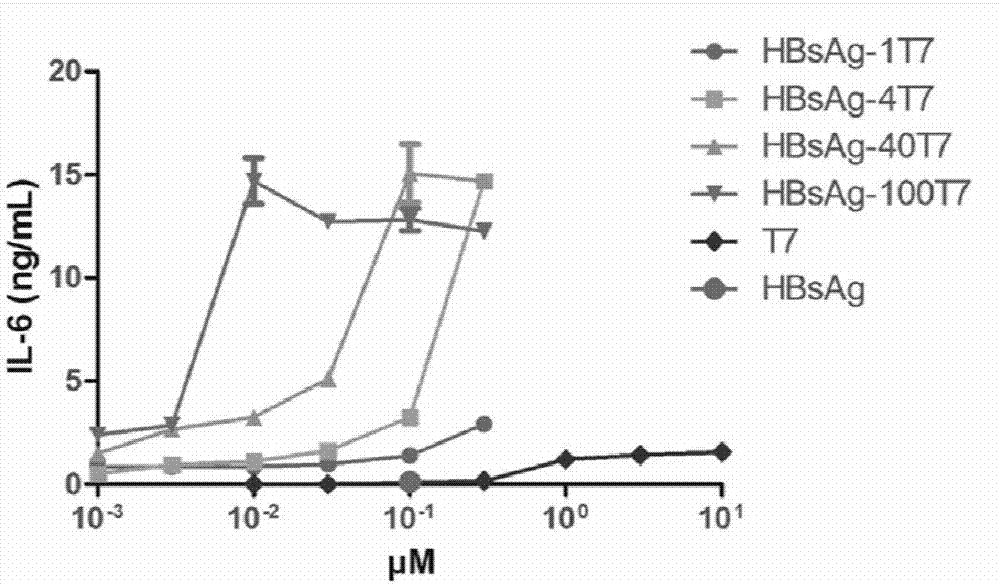
Image
Examples
Embodiment 1
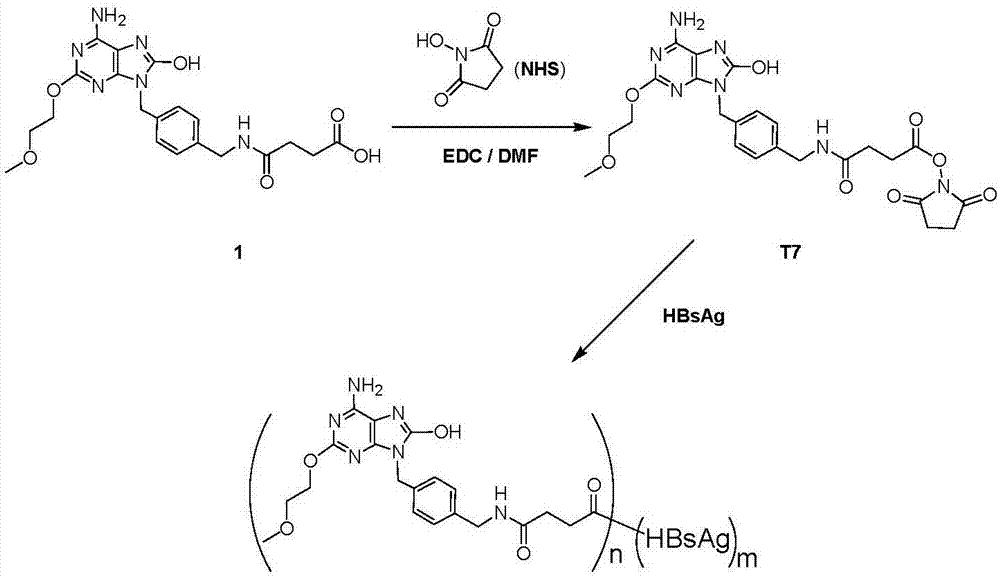
[0042] Embodiment 1: the synthesis of immunostimulant activated ester (T7)
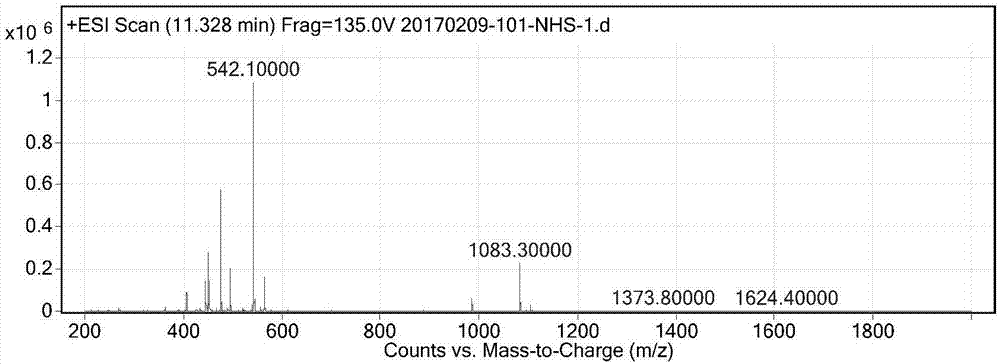
[0043] Such as figure 1 As shown, 1g of compound 1 (2.25mmol) was dissolved in 12ml of anhydrous DMF, EDC (518mg, 2.7mmol) and NHS (337mg, 2.93mmol) were added in batches, and reacted at room temperature for 2h, LC-MS detected the reaction, and the reaction was completed Finally, the reaction solution was poured into 100 ml of ice water, filtered with suction, washed with water, and dried to obtain 0.9 g of a white solid. Such as figure 2 Shown, ESI-MS: m / z=542.10[M+H]+.
[0044] It should be noted that EDC is a water-soluble carbodiimide with the chemical formula C 8 h 17 N 3 .HCl, specifically 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; NHS is N-hydroxysuccinimide; DMF dimethylformamide.
Embodiment 2
[0046] Dissolve 1 g of compound 1 (2.25 mmol) in 13.4 ml of anhydrous DMF, add 600 mg of EDC and 400 mg of NHS in batches, react at room temperature for 4 hours, and detect the reaction by LC-MS. After the reaction, pour the reaction solution into 100 ml of ice water, and filter with suction. Washed with water and dried to obtain 0.98g of white solid. ESI-MS: m / z=542.10[M+H]+.
Embodiment 3
[0048] Dissolve 1 g of compound 1 (2.25 mmol) in 10 ml of anhydrous DMF, add 450 mg of EDC and 250 mg of NHS in batches, react at room temperature for 1.5 h, and detect the reaction by LC-MS. After the reaction, pour the reaction solution into 100 ml of ice water, and filter with suction. Washed with water and dried to obtain 0.86g of white solid. ESI-MS: m / z=542.10[M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com