T cell for expressing immunosuppressive checkpoint receptor molecule and application of T cell
A technology of immunosuppression and receptor molecules, which is applied in the field of biomedicine, can solve the problems of clinical application uncertainty, cytokine storm, accidental injury of normal cells, etc., and achieve the effect of enhancing anti-tumor ability, strengthening ability, and avoiding excessive activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 lentiviral vector
[0056] In this embodiment, at first the following nucleic acid molecules are genetically synthesized:
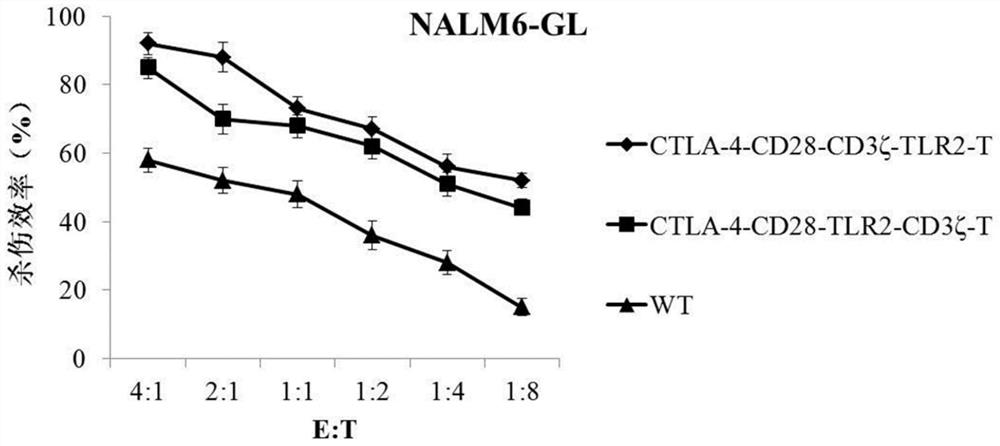
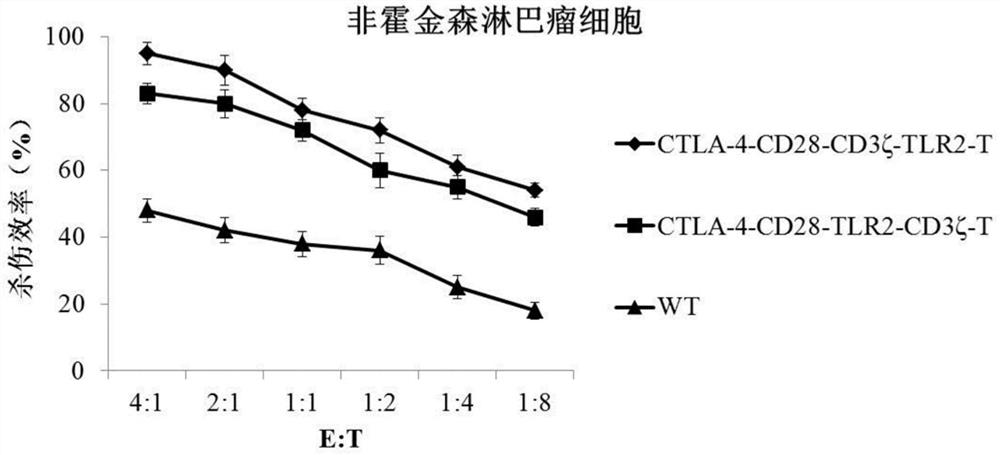
[0057] ①A receptor molecule formed in series by the nucleic acid sequences of CTLA-4, CD28 intracellular segment, CD3ζ and TLR2 (schematic diagram as shown in figure 1 shown);
[0058] ②A receptor molecule formed by tandem nucleic acid sequences of CTLA-4, CD28 intracellular segment, TLR2 and CD3ζ;
[0059] Wherein, the CTLA-4 immunosuppressive checkpoint coding gene is shown in SEQ ID NO: 3, the CD28 intracellular segment coding gene is shown in SEQ ID NO: 4, the CD3ζ coding gene is shown in SEQ ID NO: 5, and the TLR2 coding gene As shown in SEQ ID NO:6.
[0060] Adding a restriction endonuclease Pme1 restriction site and its protective bases and a restriction endonuclease Spe1 restriction site and its protective bases to the C-terminus and N-terminal of the nucleic acid molecule;
[0061] The coding gene was do...
Embodiment 2
[0062] Example 2 lentiviral packaging
[0063] In order to introduce receptor molecules into T cells, 293T cells were used to prepare recombinant lentiviruses, and when 293T cells spread 80-90% of the bottom of a 100mm culture dish, the lentiviruses were packaged:
[0064] 2 hours before virus packaging, replace the medium with DMEM containing 1% fetal bovine serum, and add 6mL / 100mm culture dish;
[0065] Prepare the plasmid mixture shown in Table 1, the pWPXLd-expression plasmid includes the lentiviral vector containing the coding gene of CTLA-4-CD28-CD3ζ-TLR2, or the lentiviral vector containing the coding gene of CTLA-4-CD28-TLR2-CD3ζ Viral vector, the pWPXLd-eGFP plasmid is an empty vector that does not contain the gene encoding the receptor molecule;
[0066] Table 1
[0067]
[0068] Add 36 μg PEI to another 500 μL opti-MEM medium, mix well, and let stand at room temperature for 5 minutes;
[0069] Mix the plasmid mixture shown in Table 1 with PEI, mix well by pip...
Embodiment 3
[0074] Example 3 T cell activation and lentiviral transfection
[0075] Peripheral blood mononuclear cells (PBMC) were separated from whole blood using Ficoll density gradient centrifugation kit (GE Company), and after red blood cells were removed, T cells were sorted out using MACS Pan-T magnetic beads;
[0076] The sorted T cells were diluted with medium (AIM-V medium + 5% FBS + penicillin 100 U / mL + streptomycin 0.1 mg / mL) to a cell concentration of 2.5×10 6 pcs / mL for use;
[0077] CD2 / CD3 / CD28 T cell activation expansion kit (Miltenyi) was used to activate T cells and placed at 37°C, 5% CO 2 The incubator was stimulated for 48 hours;
[0078]After T cells were activated for 48 hours, demagnetize the beads, centrifuge at 300g for 5 minutes, remove the supernatant, resuspend T cells with fresh medium, add different lentiviruses (MOI=10), and add 8μg / mL polybrene and 300IU / mL IL -2, placed at 37°C, 5% CO 2 incubator cultivation;
[0079] After 24 hours, centrifuge at 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com