A method for improving inclusion performance of a pharmaceutical adjuvant beta-cyclodextrin
A technology of excipient package and cyclodextrin, which is applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations of non-effective ingredients, etc., can solve the problem of insufficient stability of inclusion compounds, low inclusion rate, and low inclusion rate of cyclodextrin and other problems, to achieve the effect of improving inclusion performance and inclusion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
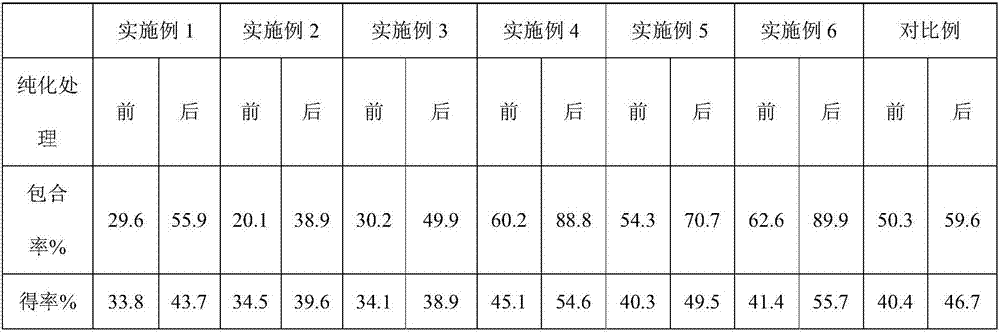
[0016] (1) Recrystallize the β-cyclodextrin produced from source A with purified water, dry at 60°C for 12 hours, and set aside; (2) Use a saturated solution method with a mixed solvent (water:[EMIM]Cl=9.5:0.5) After preparing 27mL of the saturated solution of β-cyclodextrin purified from source A, add the drug naproxen, wherein the drug naproxen and the purified β-cyclodextrin from source A are in a solid-to-solid molar ratio of 1:3 at 70 The clathrate was prepared under the condition of stirring at ℃ for 2 h; (3) The inclusion rate of the clathrate was measured by ultraviolet spectrophotometry, and the inclusion effect of the clathrate before and after purification was compared.
Embodiment 2
[0018] (1) Purify the β-cyclodextrin produced by source A with ethanol, dry it at 60°C for 12 hours, and set aside; (2) Use a saturated solution method, use 27 mL of a mixed solvent (water:[EMIM]Cl=9:1) After preparing the saturated solution of β-cyclodextrin purified from source A, add the drug naproxen, wherein the drug naproxen and the purified β-cyclodextrin from source A are prepared at a solid-to-solid molar ratio of 1:3 at 70°C The clathrate was prepared under the condition of stirring for 2 hours; 3) The inclusion rate of the clathrate was measured by ultraviolet spectrophotometry, and the inclusion effect of the clathrate before and after purification was compared.
Embodiment 3
[0020] (1) Purify the β-cyclodextrin produced by source C with acetone, dry it at 60°C for 12 hours, and set aside; (2) Use a saturated solution method, use 27 mL of a mixed solvent (water:[EMIM]Cl=8:2) After preparing the saturated solution of β-cyclodextrin purified from source C, add the drug naproxen, wherein the drug naproxen and the purified β-cyclodextrin from source C are in a solid-to-solid molar ratio of 1:3 at 70°C The clathrate was prepared under the condition of stirring for 2 hours; (3) The inclusion rate of the clathrate was measured by ultraviolet spectrophotometry, and the inclusion effect of the clathrate before and after purification was compared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com