IDT photovoltaic material and preparation method and application thereof
A photovoltaic material and dithiophene technology, applied in photovoltaic power generation, semiconductor/solid-state device manufacturing, electrical components, etc., to achieve the effects of low HOMO energy level, high stability and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of 4-fluorophenyl-[2.1.3]benzoselenodiazole-introduced dithiophene (M1) includes the following steps:
[0040] Step 1) Under argon protection, add compound 1 (3.13g, 5.24mmol), 2-(tri-n-butyl)furan (4.64g, 13.00mmol), and anhydrous toluene (150mL) into a 250mL three-necked flask, Then continue to add tetrakis (triphenylphosphine) palladium (Pd(PPh 3 ) 4 ) (0.74g, 0.64mmol), after completion, reflux reaction at 90°C for 48 hours. After the reaction is completed, cool to room temperature, pour the reaction mixture into water, extract 3 times with dichloromethane, dry the organic phase, filter, and remove excess organic solvent to obtain the crude compound 2 which is rinsed through a silica gel column , The eluent used in the leaching is a mixture of petroleum ether and dichloromethane with a volume ratio of 4:1 to obtain 2.31 g of orange solid compound 2, and the reaction yield is 77%;
[0041] The characteristics of the compound 2 are as follows:
[0042] TOF-...
Embodiment 2
[0063] The preparation of 4-fluorophenyl-[2.1.3]benzoselenodiazole-introduced dithiophene (M1) includes the following steps:
[0064] Step 1) Under the protection of argon, add compound 1 (3.13g, 5.24mmol), 2-(tri-n-butyl)furan (3.74g, 10.48mmol), and anhydrous toluene (150mL) into a 250mL three-necked flask, Then continue to add tetrakis (triphenylphosphine) palladium (Pd(PPh 3 ) 4 ) (0.81g, 0.70mmol), after completion, reflux at 85°C for 60 hours. After the reaction is over, cool to room temperature, pour the reaction mixture into water, extract 3 times with dichloromethane, dry the organic phase, filter, and remove excess organic solvent to obtain a crude product of compound 2. The crude product of compound 2 is leached through a silica gel column Washing, the washing liquid used for washing is a mixture of n-hexane and dichloromethane with a volume ratio of 3:1 to obtain 2.40 g of orange solid compound 2, and the reaction yield is 80%;
[0065] The characteristics of the compo...
Embodiment 3
[0087] The preparation of 4-fluorophenyl-[2.1.3]benzoselenodiazole-introduced dithiophene (M1) includes the following steps:
[0088] Step 1) Under argon protection, add compound 1 (3.13g, 5.24mmol), 2-(tri-n-butyl)furan (5.61g, 15.72mmol), and anhydrous toluene (150mL) into a 250mL three-necked flask, Then continue to add tetrakis (triphenylphosphine) palladium (Pd(PPh 3 ) 4 ) (0.69g, 0.60mmol), after completion, reflux reaction at 95°C for 40 hours. After the reaction is over, cool to room temperature, pour the reaction mixture into water, extract 3 times with dichloromethane, dry the organic phase, filter, and remove excess organic solvent to obtain a crude product of compound 2. The crude product of compound 2 is leached through a silica gel column Washing, the eluent used for the leaching is a mixture of petroleum ether and chloroform with a volume ratio of 5:1 to obtain 2.10 g of orange solid compound 2, and the reaction yield is 70%;
[0089] The characteristics of the comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
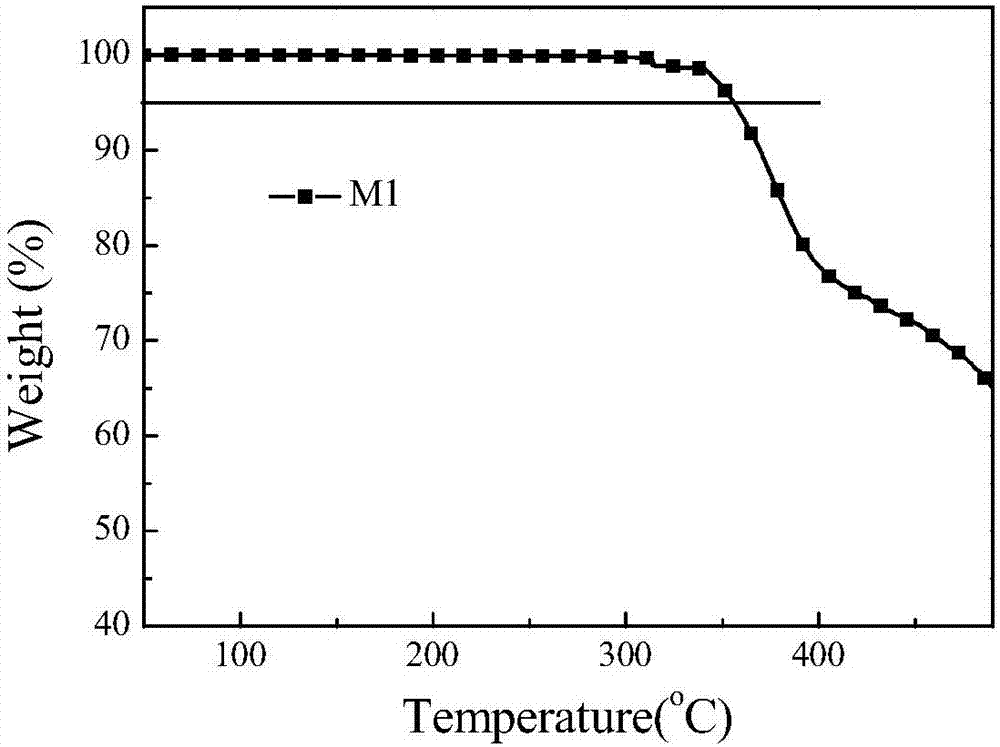
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com