Preparation method of 6-dehydronandrolone acetate
A technology of nandrolone acetate and diacetate, applied in the directions of steroids, organic chemistry, etc., can solve the problems of many synthesis steps of steroids, difficult separation and purification, complicated reactions, etc., and achieves environmental friendliness and simple operation process. , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] General reaction formula of the present invention is
[0022]
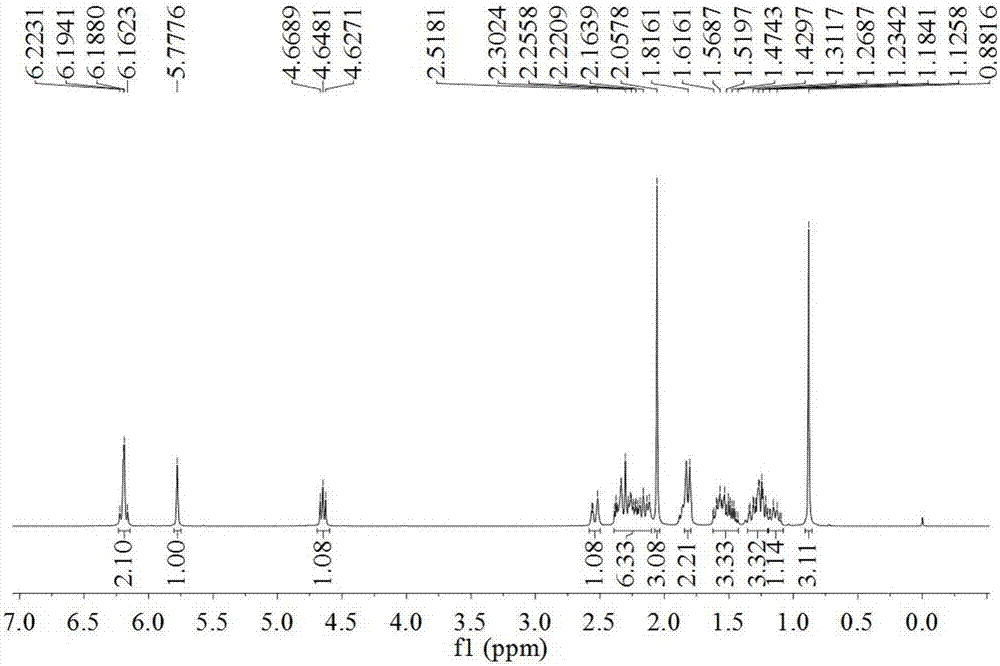
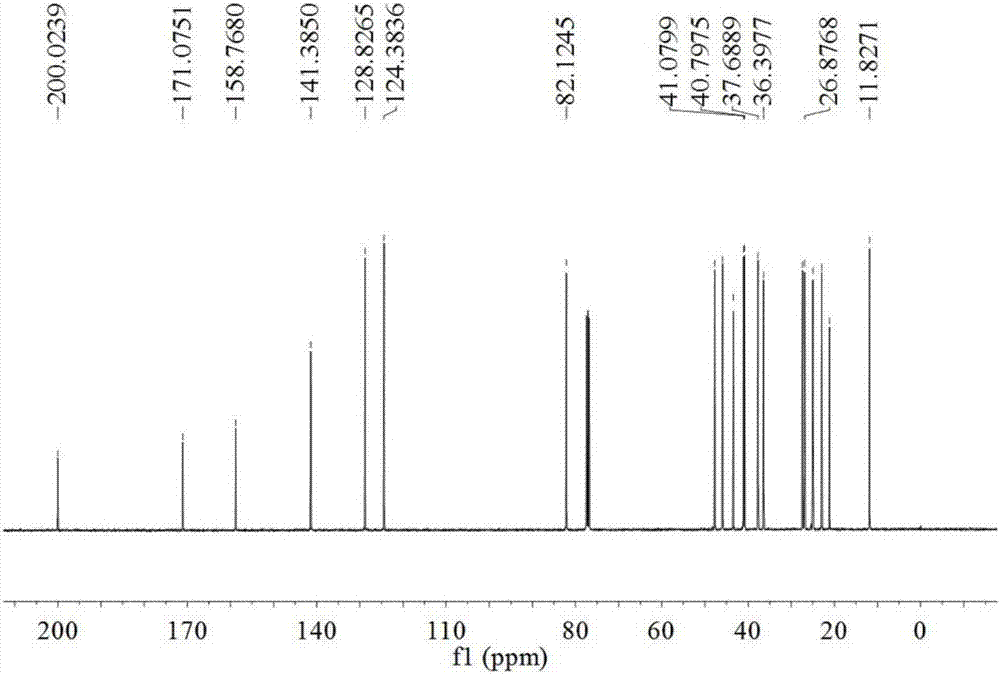
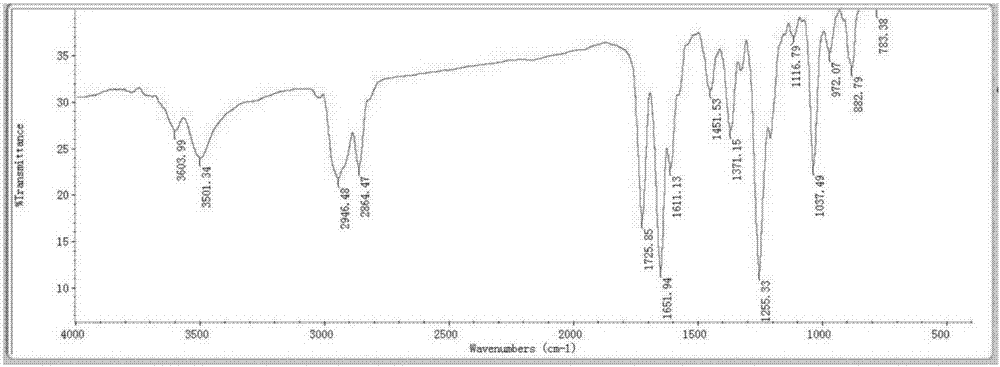
[0023] The present invention puts a No. 8 magnet in a 100mL three-necked flask, and dissolves the compound 3,5-estradiene-3,17β-diacetate (1g) in DMF (3.78g) and water (62mg) In the mixed solution, maintain the reaction temperature of the system at -10°C to -5°C. At this temperature, a solution of NBS (0.535 g) dissolved in DMF (1.65 g) was added dropwise while maintaining the reaction temperature below 0°C. After the feeding was completed, the mixture was heated to 25° C. within 30 min, and the reaction was detected by TLC. After the reaction is completed, first add sodium carbonate (0.495g) to the system, and then add sodium bromide (0.255g) to the system after the reaction is complete. React for half an hour, and then within one hour, gradually raise the temperature to 80°C, and continue the reaction for 3 hours until the end of the reaction. Stop heating after the reaction is over, cool the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com