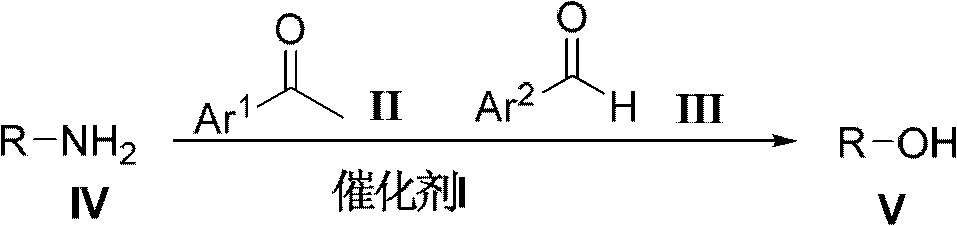
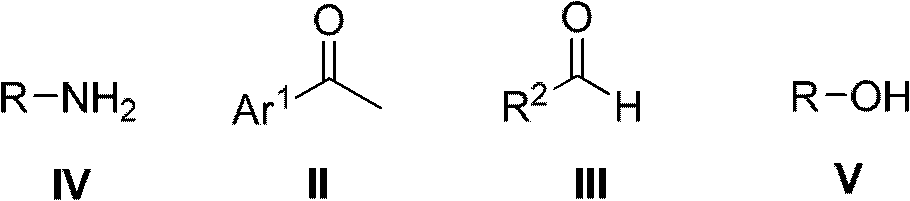
Method for preparing alcohol compounds by amine compounds
A technology for amine compounds and aldehyde compounds, which is applied in the field of preparing alcohol compounds from amine compounds, achieves the effects of simple operation process, reduced energy consumption, and avoidance of strong acid systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add acetophenone (48.00g, 0.40mol) in 500mL there-necked flask, benzaldehyde (21.20g, 0.20mol), isopropylamine (11.80g, 0.20mol) and aluminum chloride (1.40g, 10.00mmol), in The reaction was carried out at 30°C without solvent, followed by TCL. After the reaction was completed, it was filtered, and the filtrate was distilled to obtain the corresponding product isopropanol, which was detected by gas chromatography with a purity of 98% and a yield of 80%.
Embodiment 2
[0037] Add acetophenone (72.00g, 0.60mol) in 500mL there-necked flask, benzaldehyde (21.20g, 0.20mol), n-butylamine (14.60g, 0.20mol) and L-proline trifluoromethanesulfonate ( 2.64g, 10.00mmol), reacted under the reflux condition of ethanol (100.00mL), followed by TCL. After the reaction was completed, the product was filtered, and the filtrate was rectified to obtain the corresponding product n-butanol, which was detected by gas chromatography with a purity of 97% and a yield of 93%.
Embodiment 3
[0039] Add p-methylacetophenone (80.40g, 0.60mol), benzaldehyde (21.20g, 0.20mol), glycine (15.00g, 0.20mol) and diphenylamine trifluoromethanesulfonate (3.20mol) in a 500mL three-necked flask g, 10.00 mmol), reacted at 150°C under solvent-free conditions, and followed the reaction with TCL. After the reaction was completed, the product was filtered, and the filtrate was distilled to obtain the product 2-hydroxyacetic acid, which was detected by gas chromatography with a purity of 93% and a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com