Preparation method of sulfonyl phenylurea herbicide safener
A technology of sulfonylurea and herbicide, which is applied in the field of preparation of sulfonylurea herbicide safener, can solve the problems of large amount of catalyst and organic solvent, complex reaction process, serious environmental pollution, etc., and achieve the solution of environmental pollution and potential safety hazards, simplification of the reaction process, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
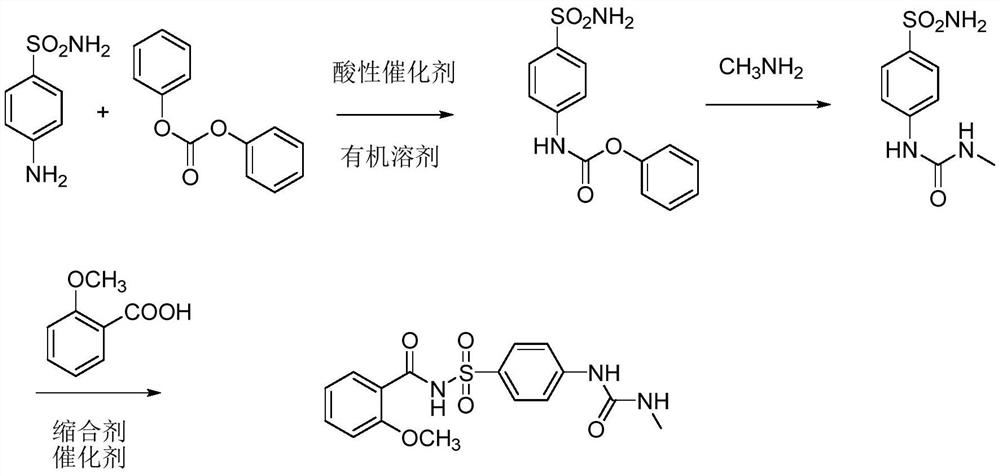
[0026] Example 1 Synthesis of phenyl 4-sulfamoylphenylcarbamate
[0027] Add p-aminobenzenesulfonamide (50g, 0.29mol), diphenyl carbonate (65.3g, 0.30mol), isobutyric acid (2.6g, 0.029mol), methanol (500ML) into the reaction flask, and heat to 65°C , reflux reaction for 48 hours, cooled to room temperature after the reaction, and filtered to obtain 72.1 g of phenyl 4-sulfamoylphenylcarbamate, yield 85.1%, melting point: 220.3-222.8°C.
example 2
[0028] Example 2 Synthesis of 4-(3-methylureido)benzenesulfonamide
[0029] Add 4-sulfamoylphenylcarbamate phenyl ester (30 grams, 0.1mol) and ethanol (200ML) into the reactor, heat to 78°C, add methylamine ethanol solution dropwise, react at 78°C for 6 hours, and react After cooling to room temperature, 21.5 g of 4-(3-methylureido)benzenesulfonamide was obtained by filtration, with a yield of 93.8% and a melting point of 210.8-212.7°C.
example 3
[0030] Example 3 Synthesis of 2-methoxy-N-{[4-(3-methylureido)phenyl]sulfonyl}benzamide
[0031] Add 4-(3-methylureido)benzenesulfonamide (15 grams, 0.065mol), o-methoxybenzoic acid (11.9 grams, 0.08mol), 4-dimethylaminopyridine (0.8 grams, 0.007mol), dicyclohexylcarbodiimide (27.0g, 0.13mol), N,N-dimethylformamide (200ML), reacted at -10°C for 36 hours, filtered, recovered the organic solvent, and weighed with ethanol 20.9 g of the product 2-methoxy-N-{[4-(3-methylureido)phenyl]sulfonyl}benzamide was obtained by crystallization, the yield was 88.2%, and the melting point was 236.2-238.4°C.
[0032] 1 H NMR (300MHz, DMSO) δ11.70(s, 1H), 9.11(s, 1H), 7.83(d, J=8.9Hz, 2H), 7.62(d, J=8.9Hz, 2H), 7.50(m ,J=9.0,7.4,1.8Hz,1H),7.39(dd,J=7.6,1.7Hz,1H),7.12(d,J=8.3Hz,1H),6.99(t,J=7.5Hz,1H) ,6.24(q,J=4.3Hz,1H),3.83(s,3H),2.66(d,J=4.6Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com