Preparation method of atomic dispersion non-noble metal fuel battery cathode catalyst
A fuel cell cathode and non-precious metal technology, applied in the field of electrochemical catalysis, to achieve the effect of simple and practical method, excellent catalytic activity and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
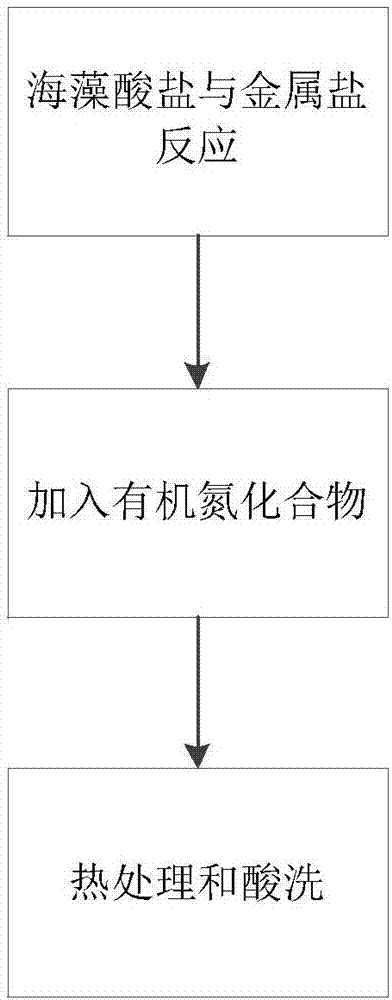
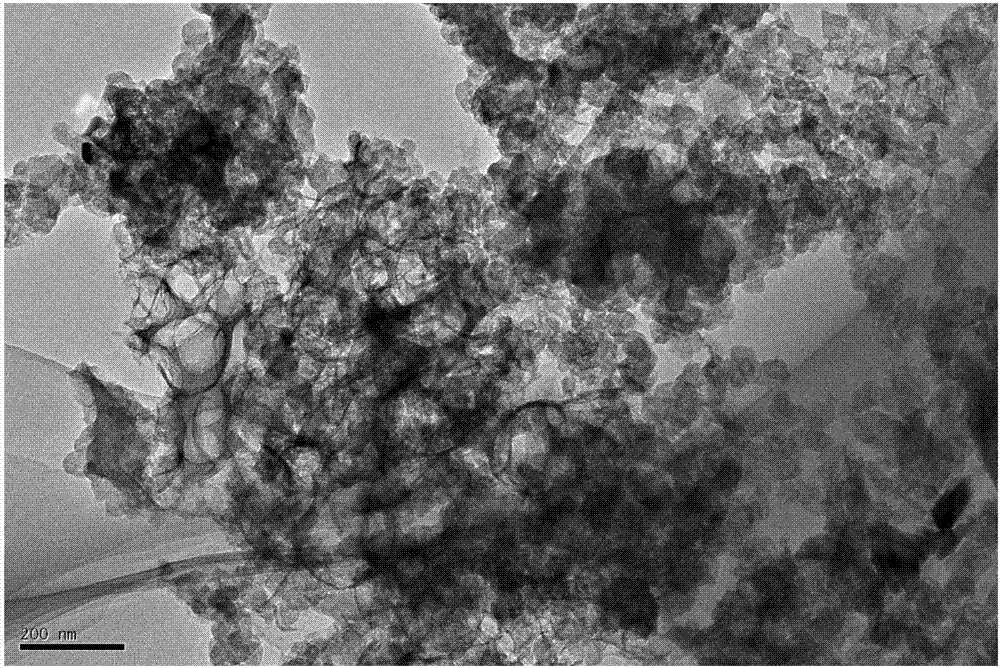
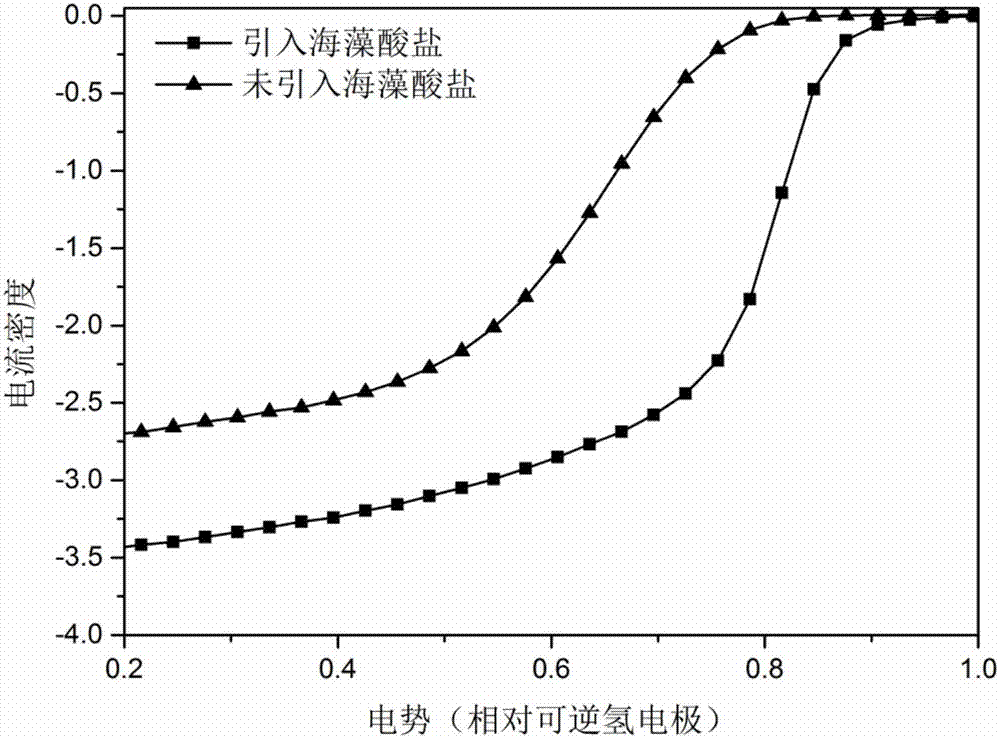
[0029] Weigh 0.0015mol of alginate and dissolve it in water at 80°C. After forming a uniform and transparent solution, add 0.006mol of FeCl 3 Iron salt, forming a flocculent chelate. After stirring for 10 hours, add 0.006mol of nitrogen precursor ethylenediamine, stir for another 48 hours, and then evaporate to dryness in an open container to obtain a powdery catalyst, and then put it into a tube Carry out heat treatment in the furnace at 700°C, feed nitrogen as a protective gas, heat up at a rate of 5°C / min, keep warm for 2 hours, take it out and put it in 0.5M sulfuric acid for pickling at 100°C for 12 hours, take it out and vacuum dry it for 12 hours , and then put it into the furnace and heat-treat as above to obtain the final catalyst.
Embodiment example 2
[0031] Weigh 0.099mol of alginate and dissolve it in water at 50°C. After forming a uniform transparent solution, add 0.006mol of Co(NO3 ) 3 Salt, forming a flocculated chelate, after stirring for 10 hours, add 0.198mol of nitrogen precursor cyanamide, stir for another 48 hours, and then evaporate to dryness in an open container to obtain a powdery catalyst, which is then placed in a tube furnace Carry out heat treatment at 1200°C, feed nitrogen as a protective gas, heat up at a rate of 10°C / min, keep warm for 5 hours, take it out and put it in 0.5M sulfuric acid for pickling at 100°C for 24 hours, take it out and vacuum dry it for 10 hours, then Then put it into the furnace and heat-treat as above to obtain the final catalyst.
Embodiment example 3
[0033] Weigh 0.05025mol of alginate and dissolve it in water at 30°C. After forming a uniform and transparent solution, add 0.006mol of Fe(NO 3 ) 3 Iron salt forms a flocculent chelate. After stirring for 10 hours, add 0.102mol of nitrogen precursor dicyandiamide, stir for another 48 hours, and then evaporate to dryness in an open container to obtain a powdery catalyst, which is then placed in a tube Carry out heat treatment in the furnace at 1200°C, feed nitrogen as a protective gas, heat up at a rate of 5°C / min, keep warm for 2 hours, take it out and put it in 0.5M sulfuric acid for pickling at 100°C for 12 hours, take it out and vacuum dry it for 12 hours , and then put it into the furnace and heat-treat as above to obtain the final catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com