Dexamethasone sodium phosphate injection and preparing method thereof
A technology of dexamethasone sodium phosphate and injection, which is applied in the fields of pharmaceutical formula, drug delivery, inorganic non-active ingredients, etc. It can solve the problems of unqualified foreign matter, precipitation of insoluble matter, unstable pH value, etc., and achieve stable pH value, The effect of prolonged storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] In order to have a further understanding of the technical solution and beneficial effects of the present invention, the technical solution of the present invention and its beneficial effects will be described in detail below.
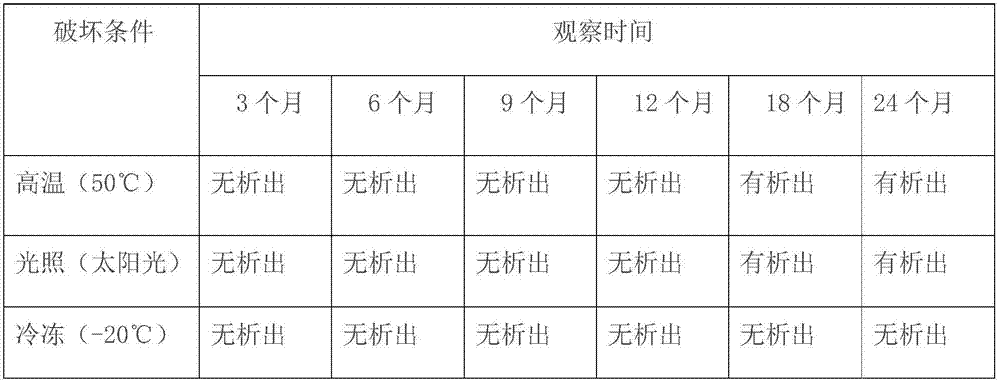
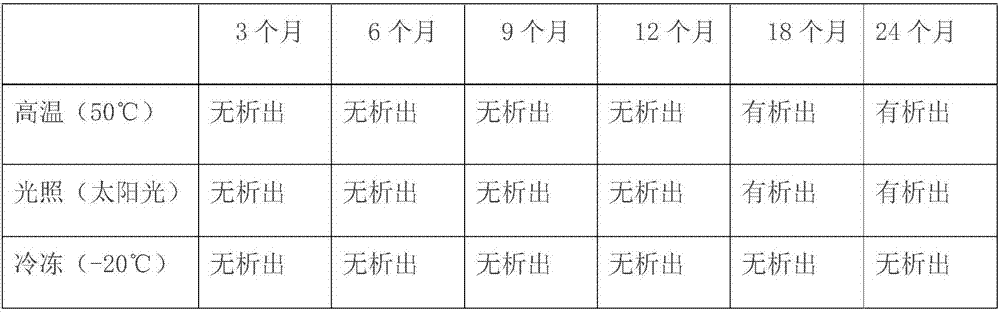
[0023] The present invention provides a kind of preparation method of dexamethasone sodium phosphate injection, mainly aimed at the phenomenon that the pH value is unstable and insolubles are separated out after the existing dexamethasone sodium phosphate injection is stored for several months, thereby The problem of unqualified visible foreign matter is caused. By adjusting the amount of propylene glycol and adding anhydrous sodium sulfite, the prepared product has excellent stability. Specifically, it includes the following steps:
[0024] S1: Add a predetermined amount of water for injection into the configuration container;
[0025] S2: adding a predetermined amount of sodium bisulfite and anhydrous sodium sulfite to dissolve them in the abov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com