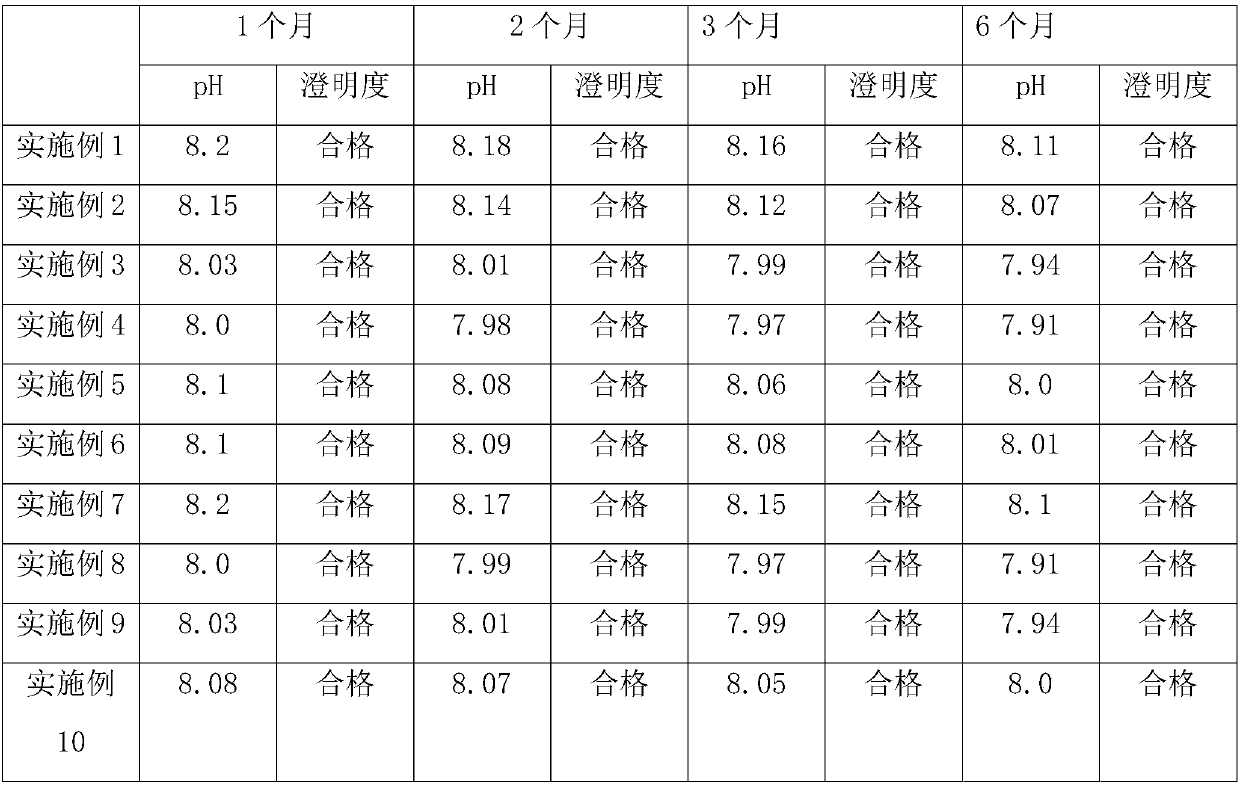
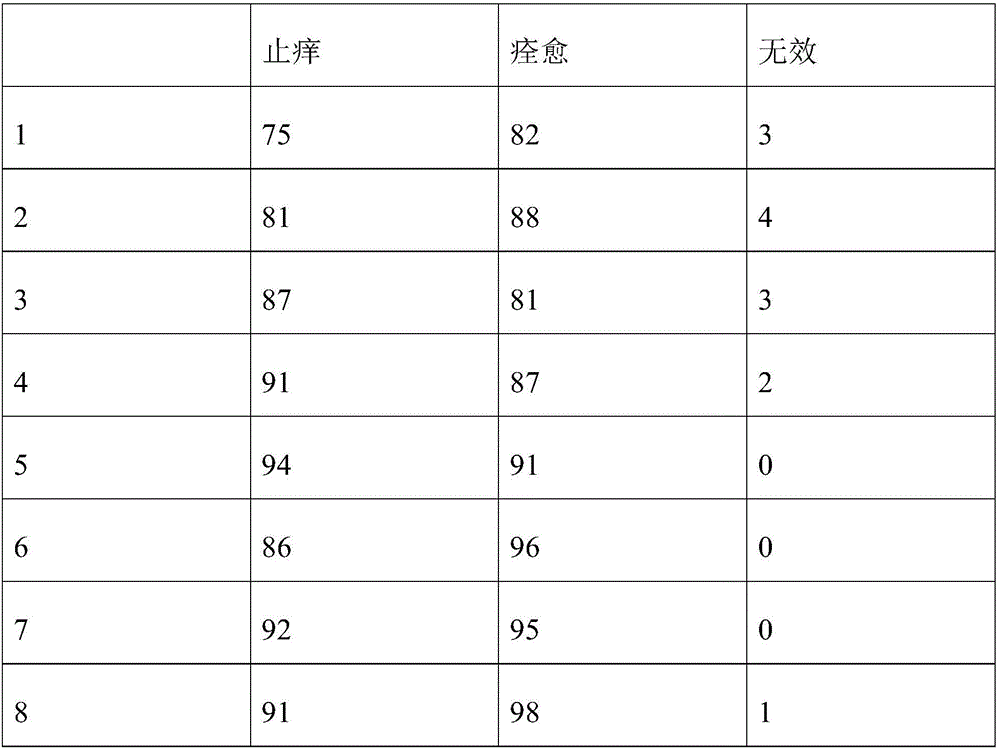
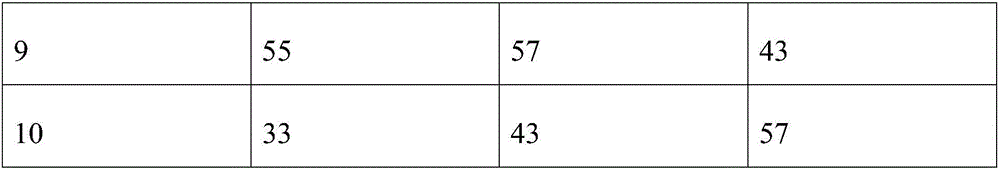
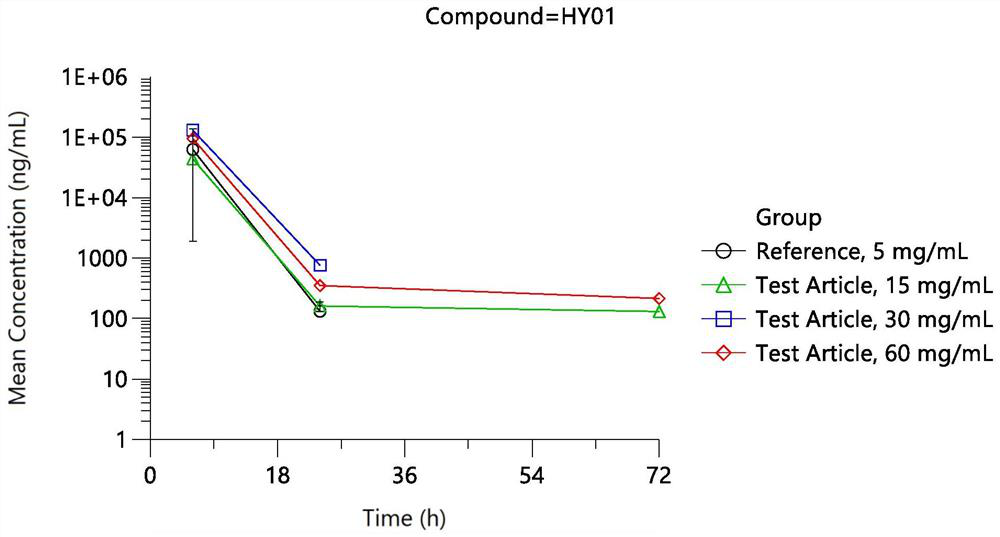
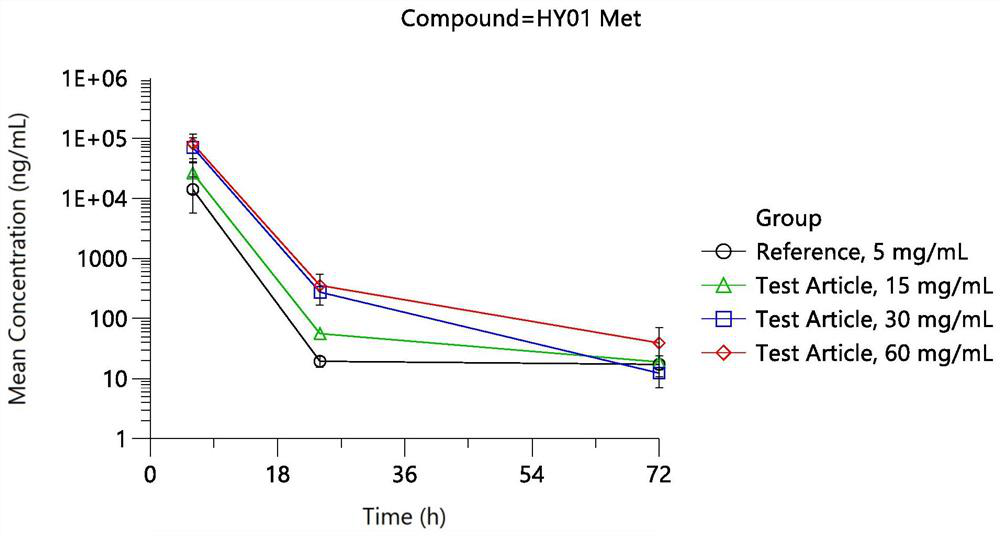
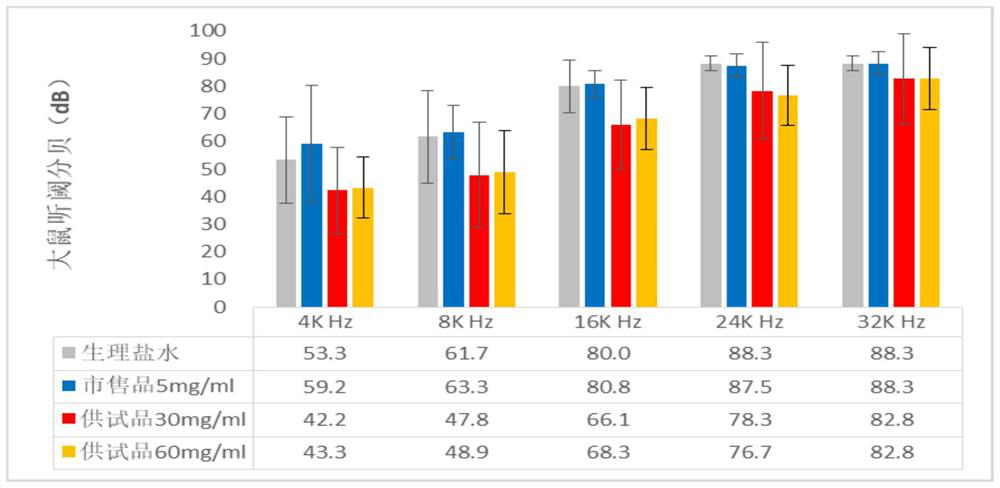
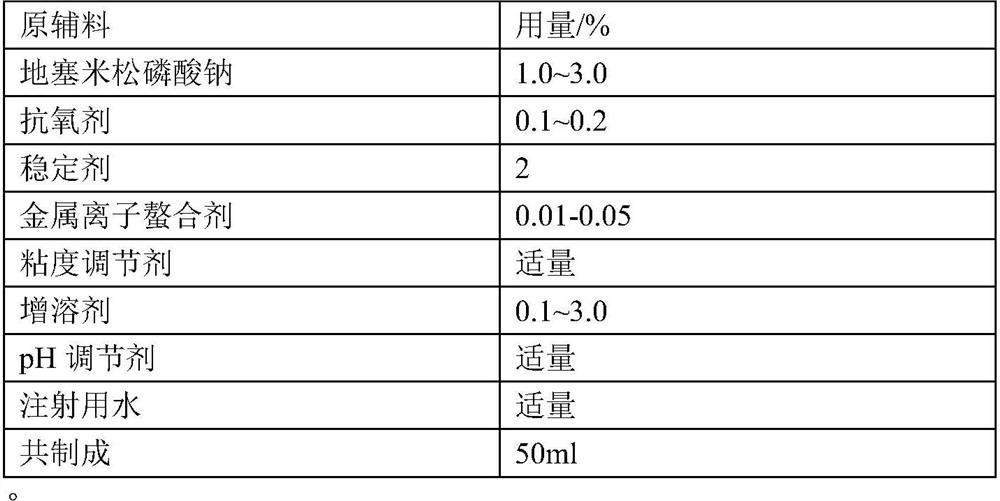
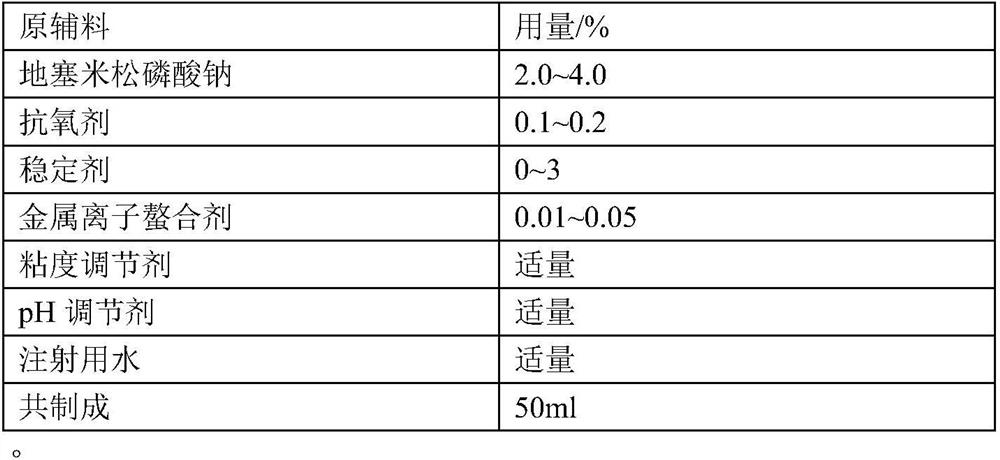
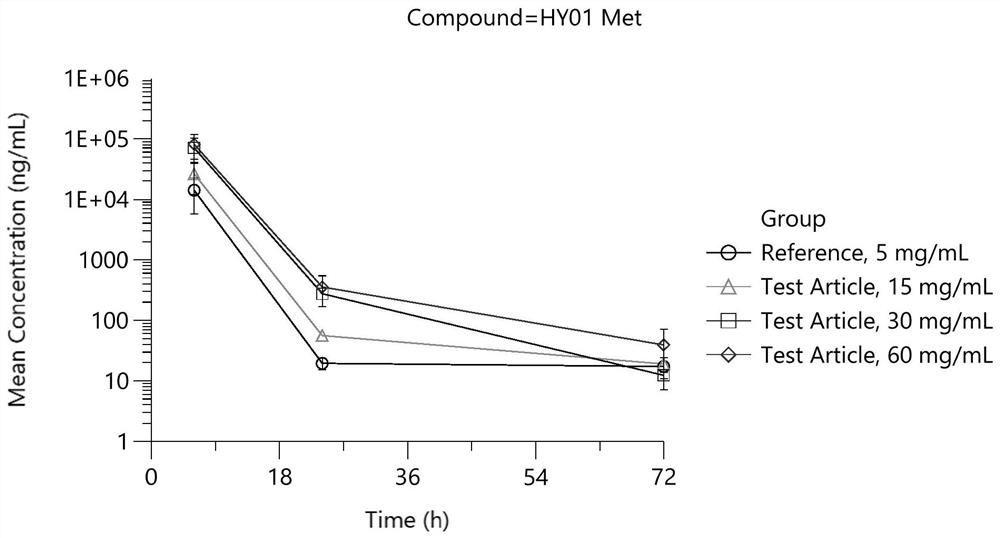
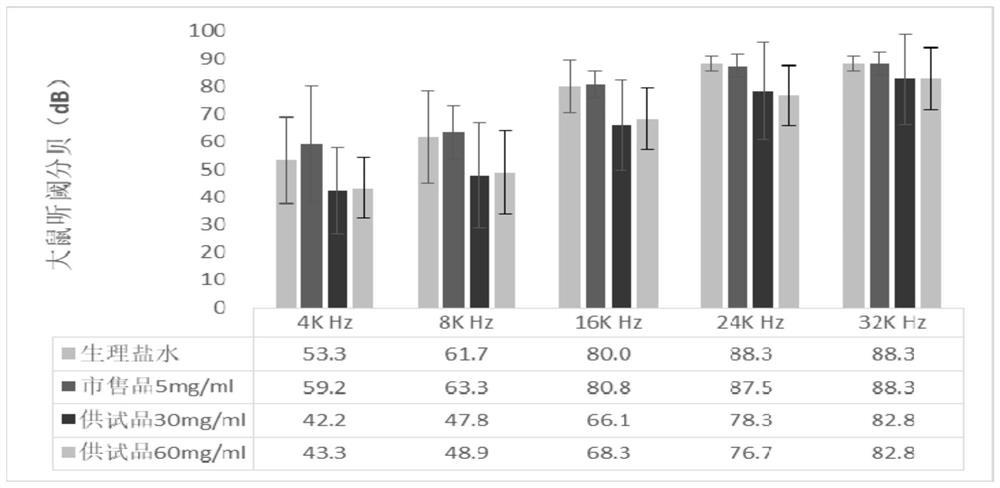
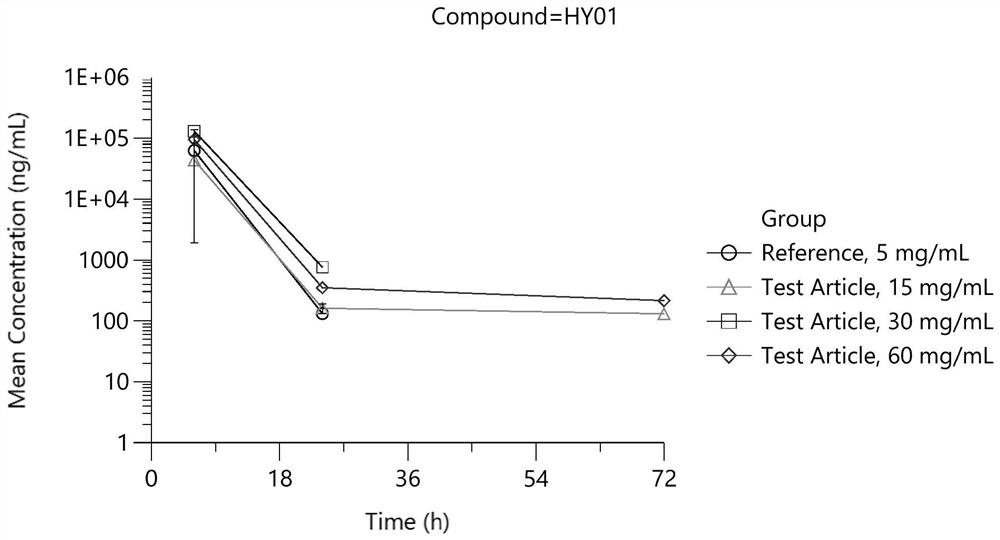
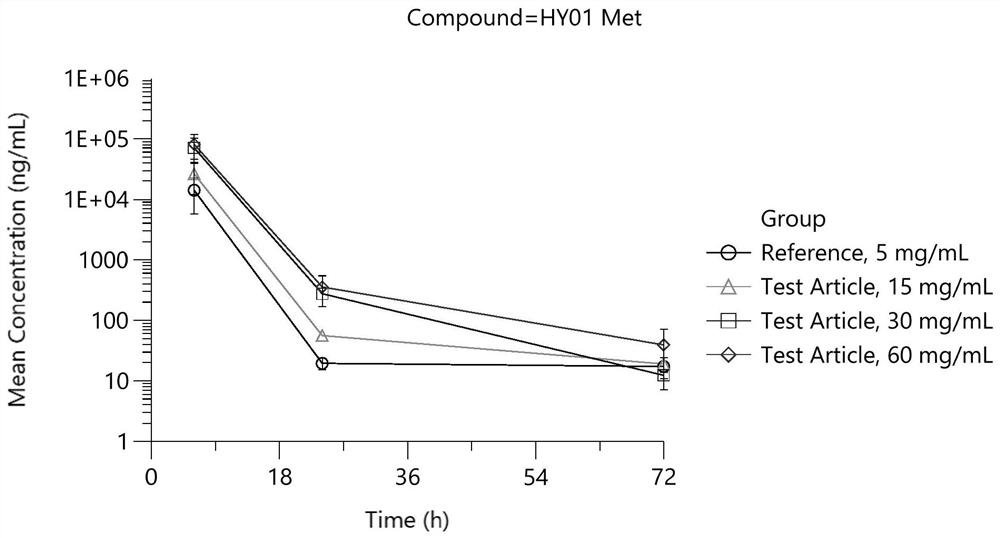
Patents
Literature
37 results about "DEXAMETHASONE SODIUM PHOSPHATE INJECTION" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
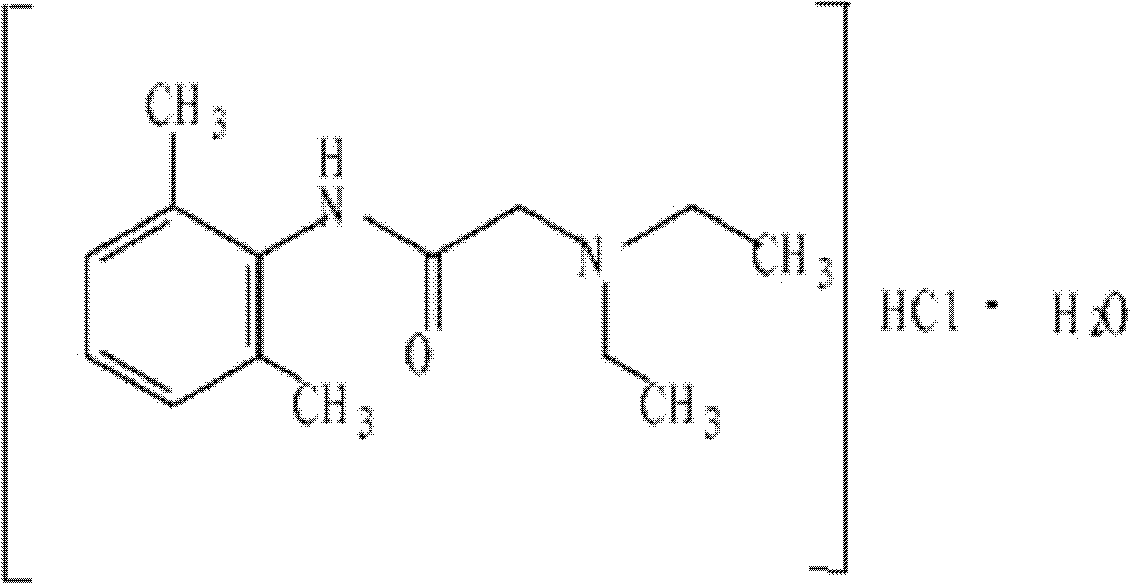
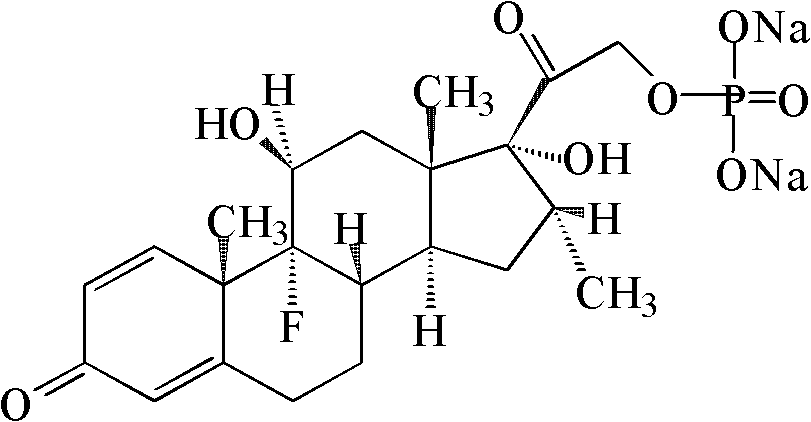
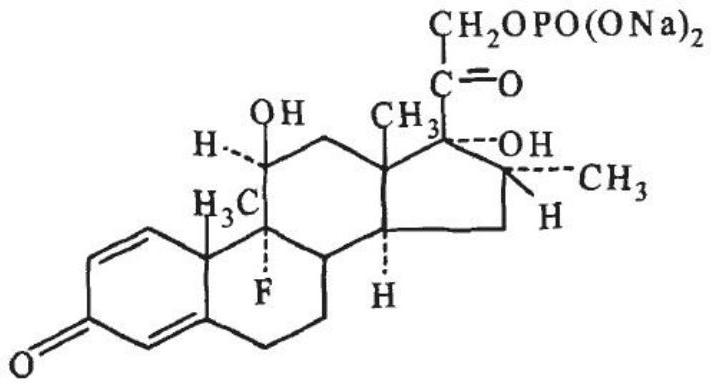
Dexamethasone Sodium Phosphate Injection, USP, is a water-soluble inorganic ester of dexamethasone which produces a rapid response even when injected intramuscularly.
Dexamethasone sodium phosphate injection
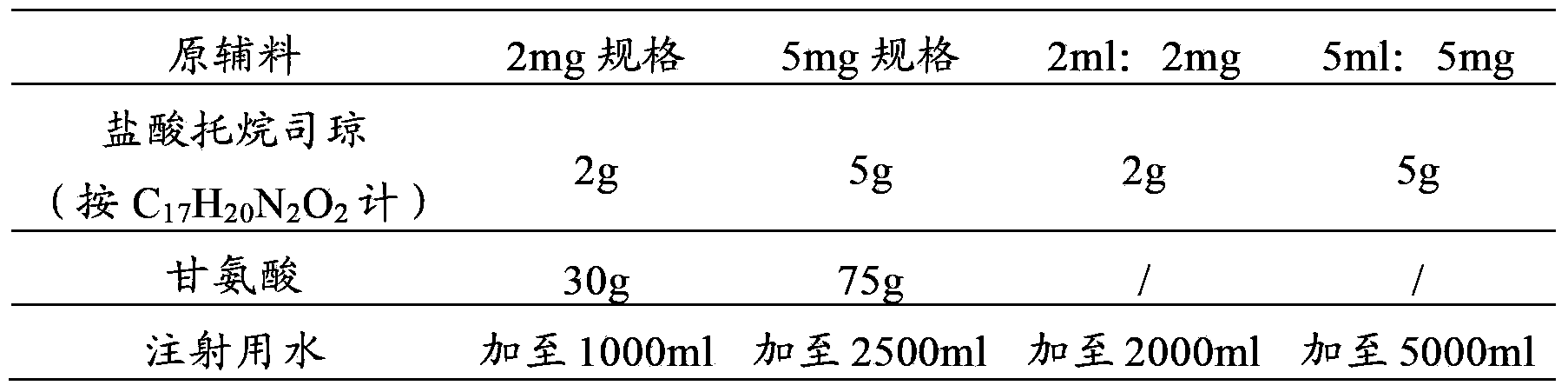
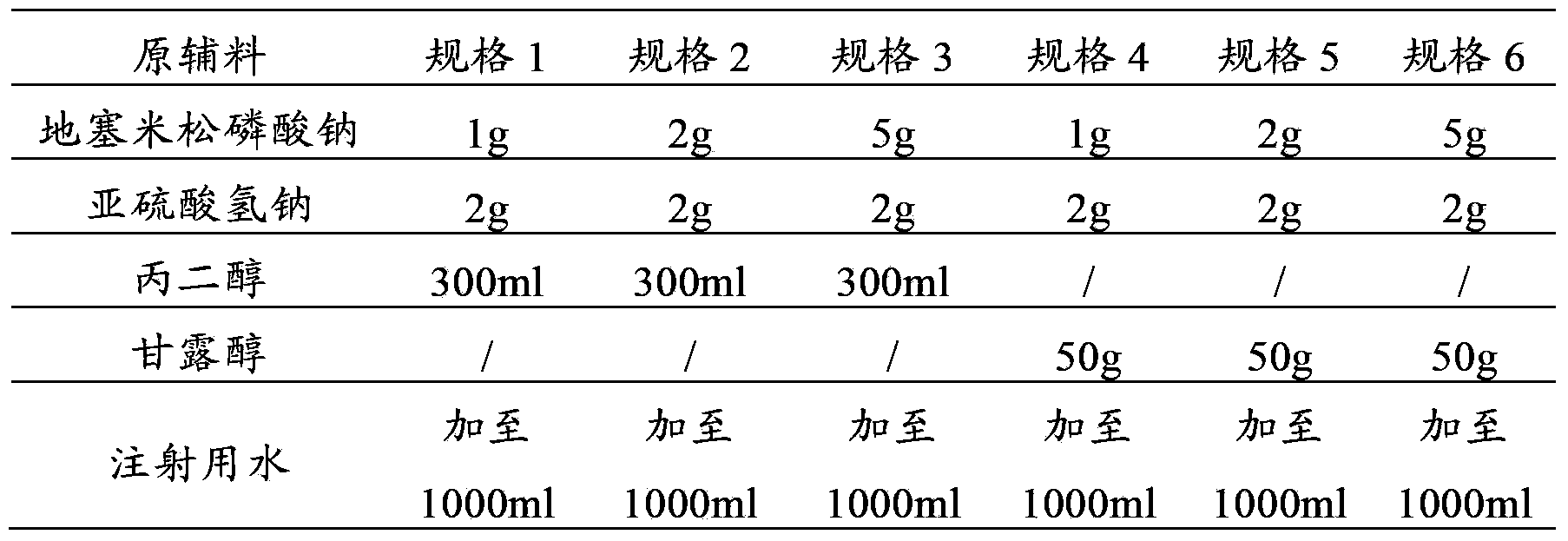
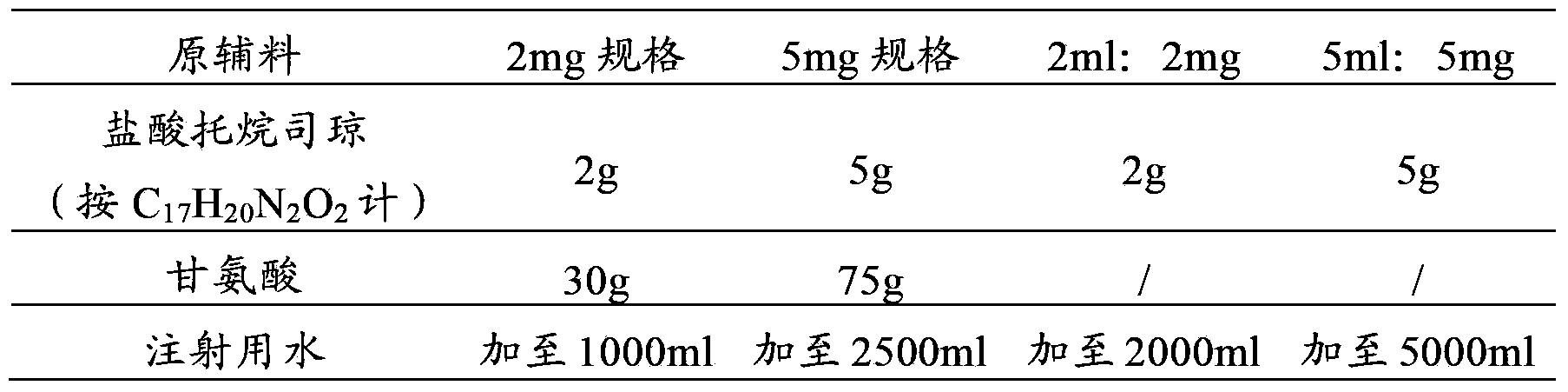
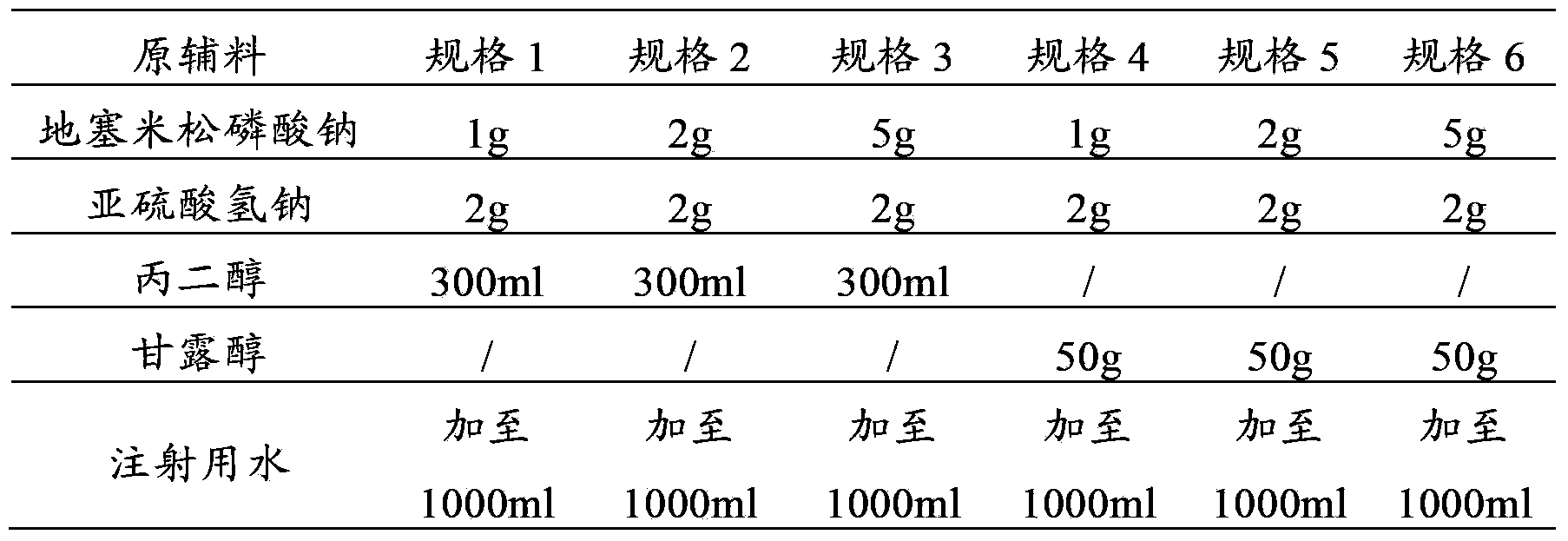
The invention provides a dexamethasone sodium phosphate injection, which consists of 0.1 to 1 percent of dexamethasone sodium phosphate, 0 to 2 percent of medicinal propylene glycol, sodium dihydrogen phosphate: disodium hydrogen phosphate=0.01 to 0.1 percent of mixed phosphate buffer according to a ratio of 0-1:10, and the balance of water for injection, wherein the used dexamethasone sodium phosphate bulk medicament preferably selects a spray drying method to produce.
Owner:TIANJIN JINYAO GRP
High-safety dexamethasone sodium phosphate injection and preparation technology thereof
ActiveCN104856946AGuaranteed stabilityReduce dosageOrganic active ingredientsPharmaceutical delivery mechanismSodium bisulfateSodium bisulfite
The invention discloses a high-safety dexamethasone sodium phosphate injection which comprises the following components: dexamethasone sodium phosphate, sodium citrate, sodium bisulfite and water for injection. In the formula of the dexamethasone sodium phosphate injection, sodium citrate is adopted to replace propylene glycol in a traditional preparation technology of dexamethasone sodium phosphate products, the dosage of sodium bisulfite is reduced, so that the stability of the injection is guaranteed, and the drug use safety is improved. The preparation technology of the dexamethasone sodium phosphate injection has the advantages that impurity generation in a production process is controlled effectively, and the drug use safety is further improved.
Owner:广东南国药业有限公司
Dexamethasone sodium phosphate injection
ActiveCN101120915AImprove product qualityReduce adverse reactionsOrganic active ingredientsAntipyreticPhosphatePropanediol
Dexamethasone sodium phosphate injection comprises the medicinal propanediol with the volume ratio of 4 percent to 6 percent, the phosphate buffer of 0.3 percent to 0.5 percent with the PH value of 8.0, the dexamethasone sodium phosphate and the injection water. Content of the dexamethasone sodium phosphate is 2mg to 5 mg per ml. The application is safe and the cost is low in the present invention.
Owner:TIANJIN PHARMA GROUP XINZHENG
Dexamethasone sodium phosphate injection
ActiveCN108403628AHigh viscosityExtended stayOrganic active ingredientsSenses disorderAntioxidantMetal ion sequestering
The invention relates to a dexamethasone sodium phosphate injection and a preparation method thereof. The injection is prepared from dexamethasone sodium phosphate, a stabilizer, an antioxidant, a metal ion chelant, a pH regulator, a viscosity regulator, a solubilizer, an osmotic pressure regulator and water for injection by dissolving and high-temperature sterilization, and has certain viscosity.The dexamethasone sodium phosphate injection is applied to intratympanic injection, and has the effects of enhancing intratympanic medicine absorption and improving sudden deafness.
Owner:和舆(苏州)医药科技有限公司
Nasal drip liquor for treating rhinitis and nasosinusitis
InactiveCN1861088ASignificant effectSymptoms improvedOrganic active ingredientsPharmaceutical delivery mechanismSinusitisTreatment effect
A kind of nasal drops for treating rhinitis and nasosinusitis is prepared from chloromycetin injection, dexamethasone-sodium phosphate injection, chlorphenamine maleate injection, ribavirin injection, metronidazole injection and lidocaine hydrochloride injection.
Owner:刘明芳
Dexamethasone sodium phosphate injection intermediate testing method
InactiveCN105136697ASolve the problem of long detection timeReduce labor costsColor/spectral properties measurementsLinear regressionLength wave
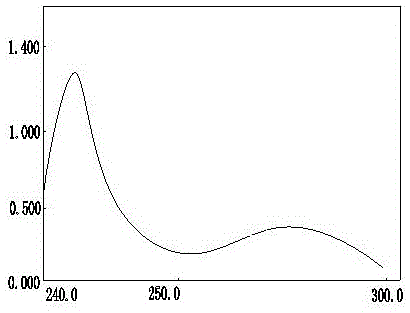
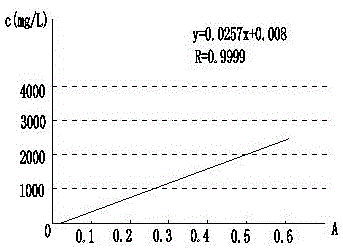
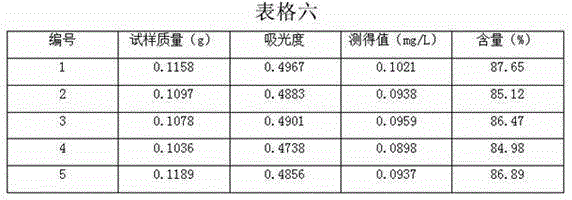
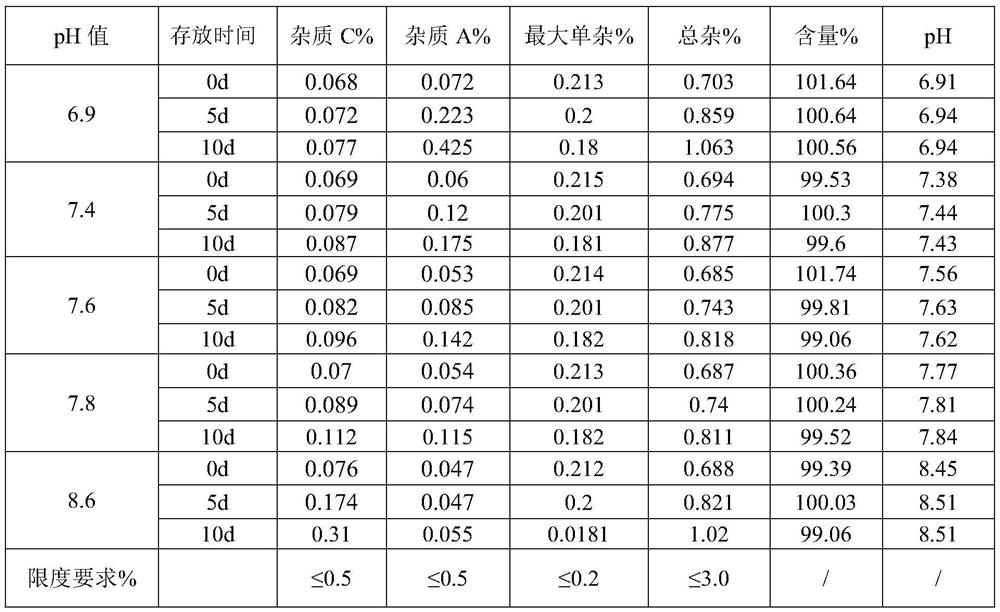
The invention discloses a dexamethasone sodium phosphate injection intermediate testing method. The dexamethasone sodium phosphate injection intermediate testing method comprises following steps: a dexamethasone sodium phosphate injection intermediate sample is dissolved in a 0.06-0.15% dimethyl sulfoxide unsaturated organic solution so as to prepare sample solutions of different concentration, wherein the concentration of the sample solutions ranges from 1000 to 3000mg / L; the sample solutions are delivered into an ultraviolet spectrophotometer for detection, a standard curve is drawn based on obtained absorbance values, and a linear regression equation is obtained according to the standard curve based on Beer-Lambert law; a sample to be tested is prepared into a solution according to the step 1, and then is subjected to absorbance value detection at a wavelength ranging from 200 to 300nm, and then the content of the dexamethasone sodium phosphate injection intermediate is calculated based on the linear regression equation. With equal labor and environment investment, yield of each batch is increased by 20%, production cost is reduced, and at the same time product yield is improved.
Owner:安徽城市药业股份有限公司
Dexamethasone sodium phosphate injection and preparation method thereof
ActiveCN112245386AGuaranteed permeabilityGuaranteed stabilityOrganic active ingredientsAntipyreticSodium phosphatesAntioxidant
The invention belongs to the field of medicines, and particularly relates to a dexamethasone sodium phosphate injection and a preparation method thereof. The dexamethasone sodium phosphate injection comprises the following components in percentage by weight of 1.04%-1.14% of dexamethasone sodium phosphate, 2.56%-2.598% of sodium citrate dehydrate, 0.007%-0.010% of citric acid and the balance of water for injection. According to the dexamethasone sodium phosphate injection and the preparation method thereof provided by the invention, the sodium citrate dehydrate and the citric acid are used asauxiliary materials, so that the safety problem caused by adding an organic solvent and an antioxidant is avoided; the osmotic pressure and the pH value of a preparation are ensured by adopting the proper sodium citrate dehydrate dosage and citric acid dosage; according to the preparation process, the stability of the preparation is ensured by adopting a relatively low liquid preparation temperature and nitrogen filling and sterilization processes, and the problem of drug stability is solved.
Owner:北京鑫开元医药科技有限公司
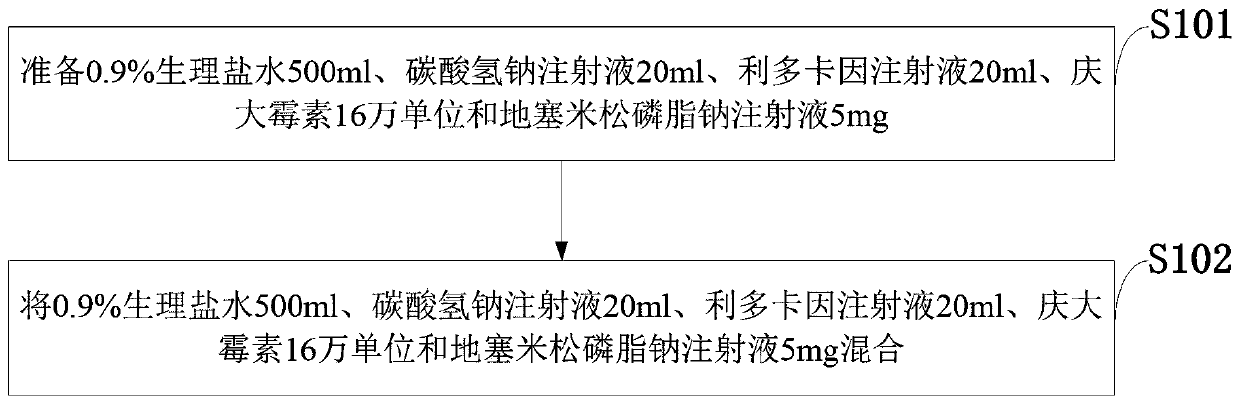
Tumor radiotherapy medicine capable of lowering damage of RE (radiation esophagitis) to patient and preparation method thereof
InactiveCN110200990ATreatment went wellShorten the timeOrganic active ingredientsDispersion deliveryDexamethasoneNursing care
The invention belongs to the technical field of clinical nursing and discloses tumor radiotherapy medicine capable of lowering damage of RE (radiation esophagitis) to a patient and a preparation method thereof. The medicine comprises 500ml of 0.9% normal saline, 20ml of sodium bicarbonate injection, 20ml of lidocaine injection, 160000 unit of gentamicin and 5mg of dexamethasone sodium phosphate injection. The tumor radiotherapy medicine has the advantages that the self-made oral liquid using sodium bicarbonate as the main raw material is used to delay the RE occurrence time of the patient, reduce RE level, shorten RE time or avoid RE so as to allow the treatment of the patient to be completed smoothly; the continuing nursing care is used to guide the self-nursing of the patient who leavesthe hospital, guide the patient to continuously follow the original diet principle, pay attention to self-monitoring and regularly have reexamination in the hospital to conveniently obtained further health promotion.
Owner:陶鹤
Technology for preparing dexamethasone sodium phosphate injection
InactiveCN105326787ASimple stepsRaw materials are easy to getOrganic active ingredientsAntipyreticDexamethasone acetateDiethyl ether
The invention relates to a technology for preparing a dexamethasone sodium phosphate injection. The technology includes the following steps that dexamethasone sodium phosphate is prepared, wherein dexamethasone acetate epoxide serves as a staring material, a dexamethasone sodium phosphate solution is obtained sequentially through a ring-opening reaction, recrystallization achieved through acetone or diethyl ether, base-catalyzed hydrolysis, pyrophosphoryl chloride esterification and a salt formation reaction achieved through neutralization, and the dexamethasone sodium phosphate solution is recrystallized to obtain a dexamethasone sodium phosphate crystal; the dexamethasone sodium phosphate crystal is subjected to ball milling to be powder after being dried; the dexamethasone sodium phosphate powder is dissolved with injection water to be diluted, and excipient and a disodium hydrogen phosphate solution are added to prepare the dexamethasone sodium phosphate injection. By means of the technology, the problems that according to an existing preparation method, recrystallized particles are small in the recrystallization process and the content of effective components of a prepared dexamethasone sodium phosphate injection is not stable are solved.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
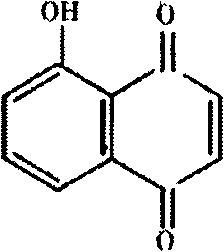
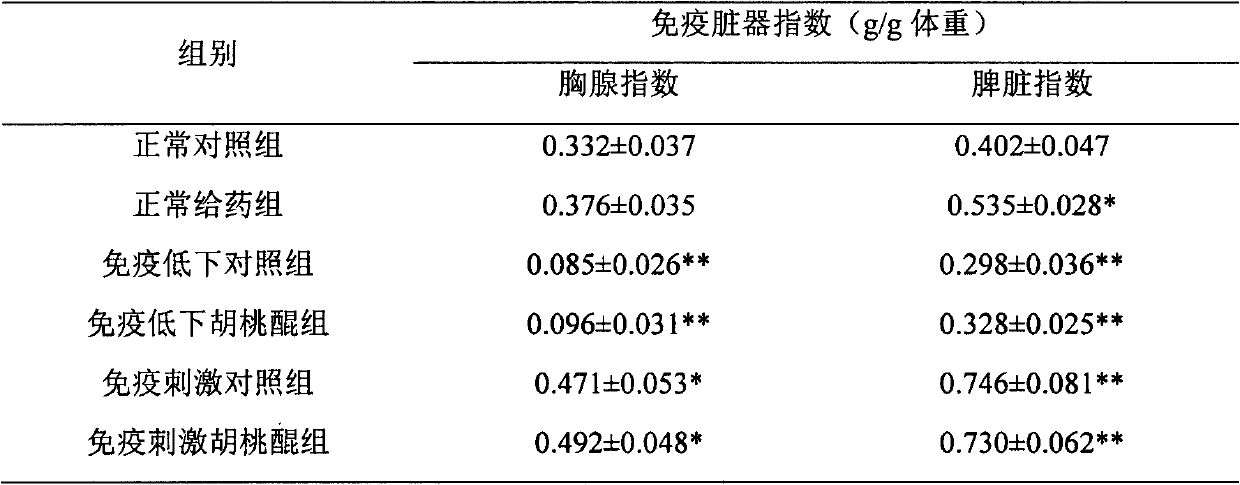
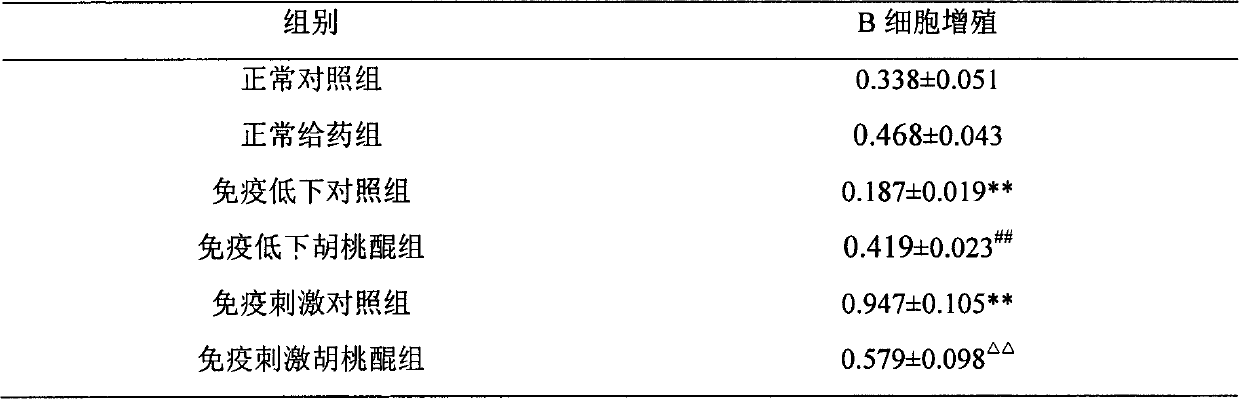
Application of juglone for adjusting immune states of mice and oxidizing reactions
InactiveCN102824335AEnhance humoral immunityRegulate immune system functionOrganic active ingredientsAntinoxious agentsOrganismImmunologic function
The invention relates to the technical field of medicines, discovers an application of juglone for adjusting immune states of mice and oxidizing reactions, and mainly researches medicinal composition juglone. A chemical name of the juglone is 5-hydroxy-1,4-naphthoquinone and 5-hydroxy-1,4-naphthalene diketone. An English name of the juglone is Juglone, walnuts quinine, 5-hydroxy-1,4-naphthoquinone and 5-hydroxynaphthalene, regianin, Nucin. A molecular formula of the juglone is C10H6O3. A relative molecular weight is 174.15. An immunocompromised model is built through systematic pharmacological tests and by using KM mice and adopting dexamethasone sodium phosphate injecta, a mouse immunostimulation model is built by using sheep red blood cells (SRBC) to induce the mice to produce SRBC immune bodies and cyclophosphamide, a fact that the juglone can recover low immunologic functions to a normal level is proved, the immunostimulation states are improved, self stability of an organism is maintained, and both-way immunoregulation functions are displayed. The juglone displays functions of adjusting organism immune system functions by adjusting oxidative stress reactions in the organism.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Pharmaceutical composition containing tropisetron hydrochloride and dexamethasone sodium phosphate
InactiveCN103393701AImprove antiemetic efficacyDigestive systemHeterocyclic compound active ingredientsFructoseRinger's solution
The invention relates to a pharmaceutical composition containing containing tropisetron hydrochloride and dexamethasone sodium phosphate, and particularly relates to a combined application package containing a tropisetron hydrochloride injection and a dexamethasone sodium phosphate injection. When the combined application package is in use, the tropisetron hydrochloride injection and the dexamethasone sodium phosphate injection are co-dissolved in normal saline, a Ringer's solution, 5% glucose injection, a fructose solution and the like for intravenous infusion, so that a curative effect of stopping vomit can be increased.
Owner:HAINAN LINGKANG PHARMA CO LTD
Compound mouthwash
PendingCN113521253AReduce incidenceReduce infection rateOrganic active ingredientsPeptide/protein ingredientsDexamethasoneOral mucous membrane
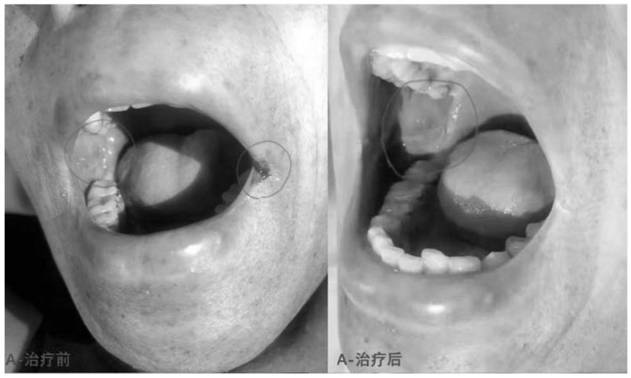
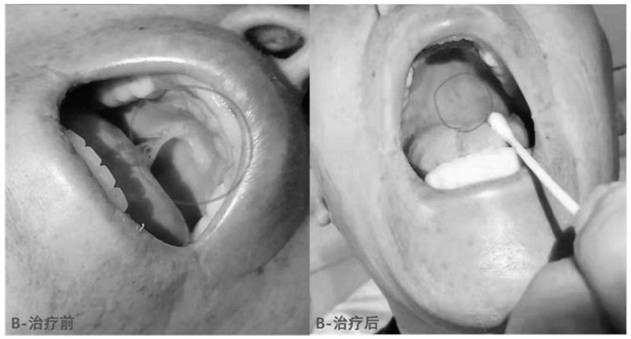
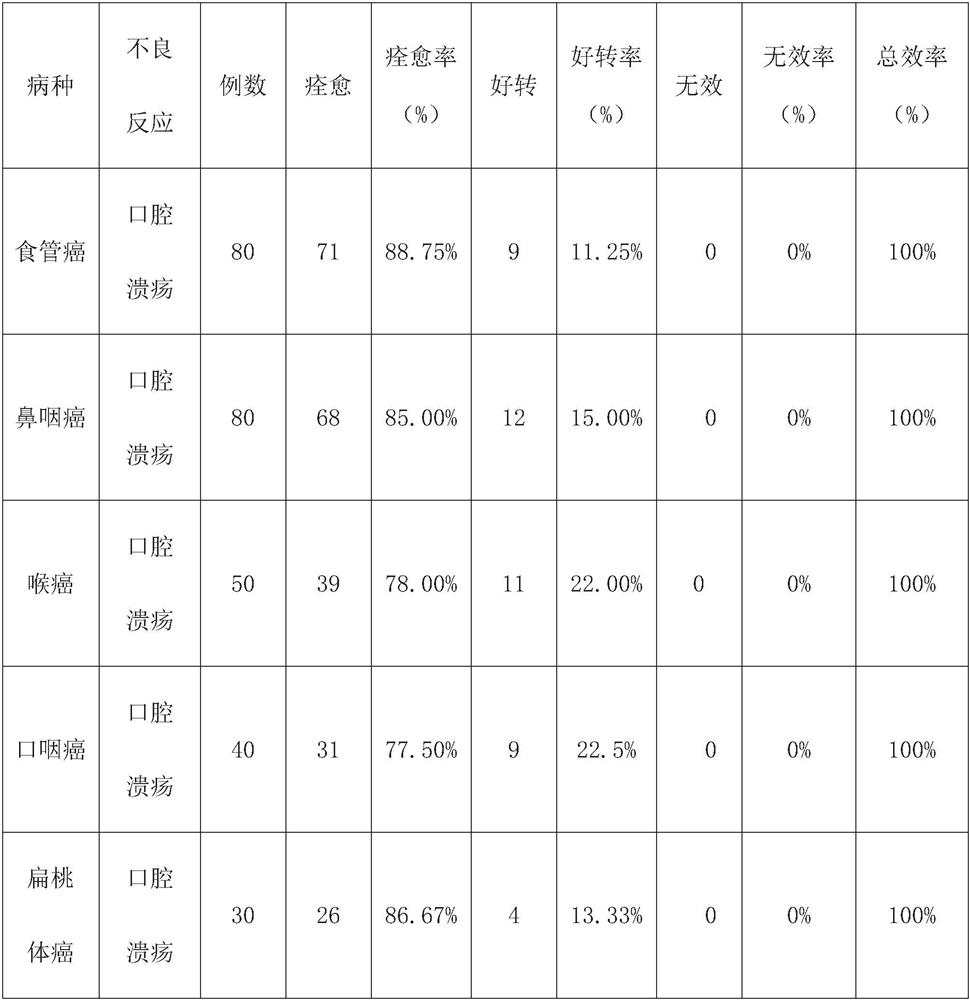
The invention discloses compound mouthwash. The compound mouthwash is prepared by adding 10mg of dexamethasone sodium phosphate injection, 113mg of recombinant human interleukin for injection and 0.4 g of lidocaine injection into 200ml of sterilizing water for injection, and uniformly mixing the materials. The compound mouthwash can reduce the incidence rate and infection rate of chemoradiotherapy-related oral mucositis and shorten the hospitalization time.
Owner:中国人民解放军西部战区总医院
Preparation method of dexamethasone sodium phosphate for injection
InactiveCN105326788ASimple stepsRaw materials are easy to getOrganic active ingredientsAntipyreticDexamethasone acetateBULK ACTIVE INGREDIENT
The invention relates to a preparation method of dexamethasone sodium phosphate for injection. The preparation method comprises the following steps that the dexamethasone sodium phosphate is synthesized, namely dexamethasone acetate epoxide is used as a starting raw material, and a dexamethasone sodium phosphate solution is obtained through ring-opening reaction, recrystallization, base-catalyzed hydrolysis, pyrophosphoryl chloride esterification and neutralization and salification reaction sequentially, and the dexamethasone sodium phosphate solution undergoes recrystallization again to obtain dexamethasone sodium phosphate crystals; the dexamethasone sodium phosphate crystals are dried and then are ball-milled into powder; the dexamethasone sodium phosphate powder is dissolved and diluted with water for injection, and an excipient and a disodium hydrogen phosphate solution are added into the solution to obtain dexamethasone sodium phosphate injection. The preparation method overcomes the problem that the dexamethasone sodium phosphate injection prepared by adopting an existing preparation method is higher in cost and unstable in active ingredient content.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
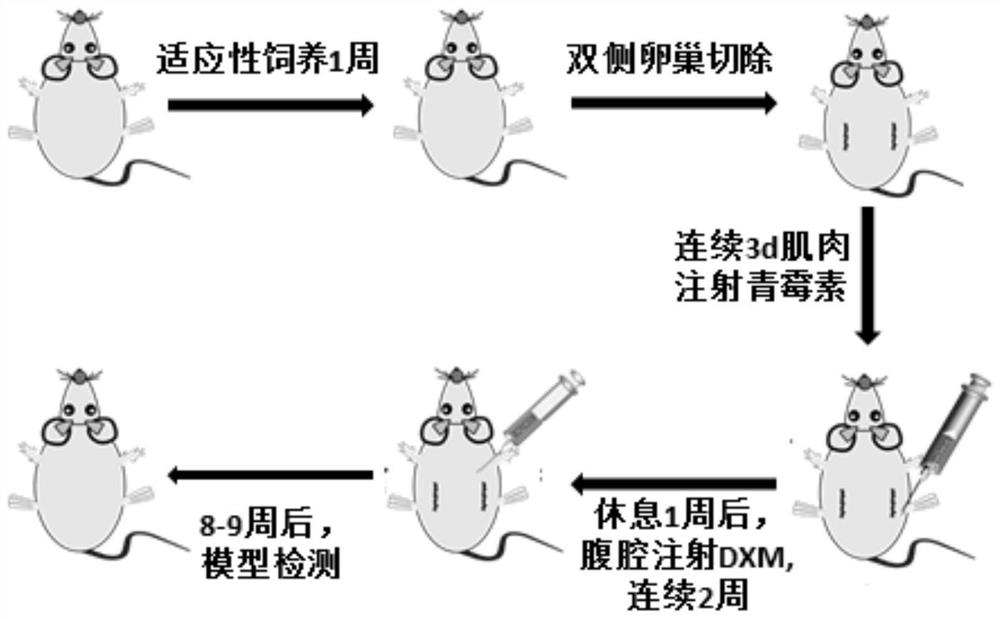
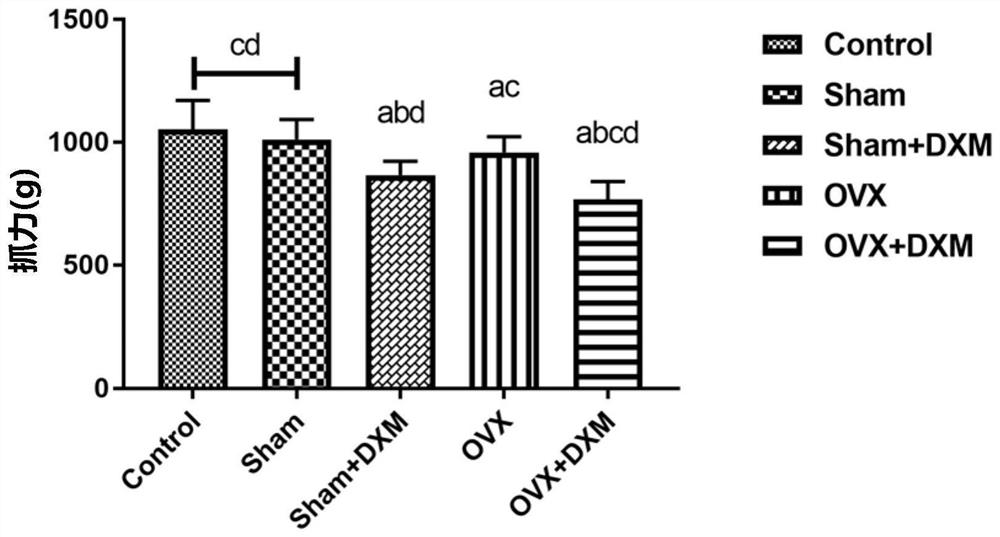
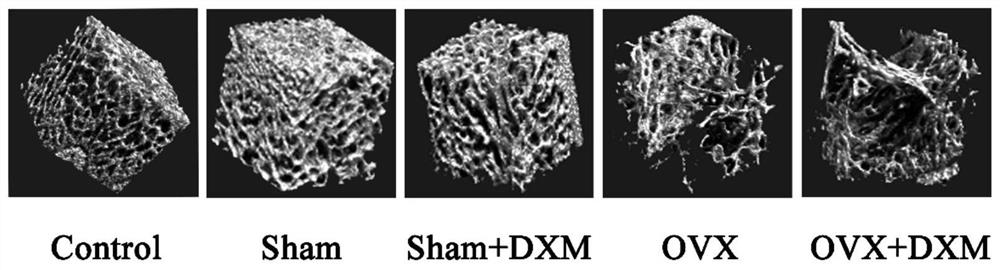
Construction method of sarcopenia- osteoporosis rat model
InactiveCN111670859AShort modeling cycleEasy to operateOrganic active ingredientsAnimal husbandryPhysiologySodium phosphates
The invention discloses a construction method of a sarcopenia-osteoporosis rat model. The method comprises the following steps of selecting female SD rats which are not pregnant and 6 months old, removing bilateral ovaries, feeding for 1 week, injecting 0.8-1.2 mg / kg / d of dexamethasone sodium phosphate injection into the abdominal cavities of the rats, and continuously injecting for 2 weeks; continuously feeding the rats for 8-9 weeks to obtain a rat model with sarcopenia-osteoporosis; meanwhile, a simple and reliable sarcopenia-osteoporosis judgment index is also determined. The composite modeling method has the advantages of being short in modeling period, stable in model, high in rat survival rate, high in operability, low in cost and the like, SP and OP can be effectively distinguished, sarcopenia-osteoporosis of human beings can be truly simulated, repeatability is high, economy and practicability are achieved, and a theoretical basis is provided for researching etiology and pathogenesis and prevention and treatment strategies of OS.
Owner:广州中医药大学第三附属医院
Foreskin phimosis debonding liquid preparation method
InactiveCN103202847AHas anti-inflammatory and anti-allergic effectsAvoid stickingOrganic active ingredientsAntipyreticForeskinAnti allergy
The invention relates to a foreskin phimosis debonding liquid preparation method. The method comprises the following steps: taking 20ml of a lidocaine hydrochloride gel, taking 1-6ml of a dexamethasone sodium phosphate injection, and fully mixing the gel and the injection. Foreskins are loosed, aches are mitigated and foreskin phimosis glans adhesion is easily solved several minutes after the medicinal liquid prepared through the method is sprayed on a male genitals. The medicinal liquid prepared through the method has anti-inflammation and anti-allergy effects.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Method for efficiently removing dexamethasone sodium phosphate crystal water
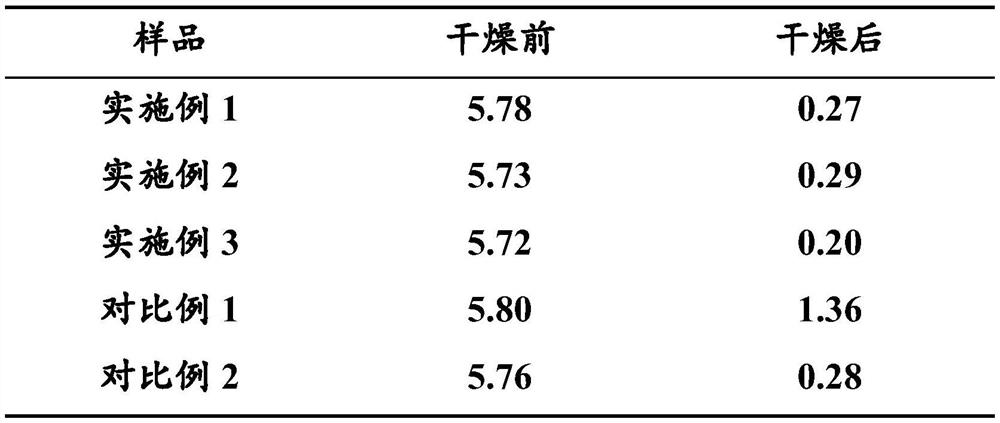
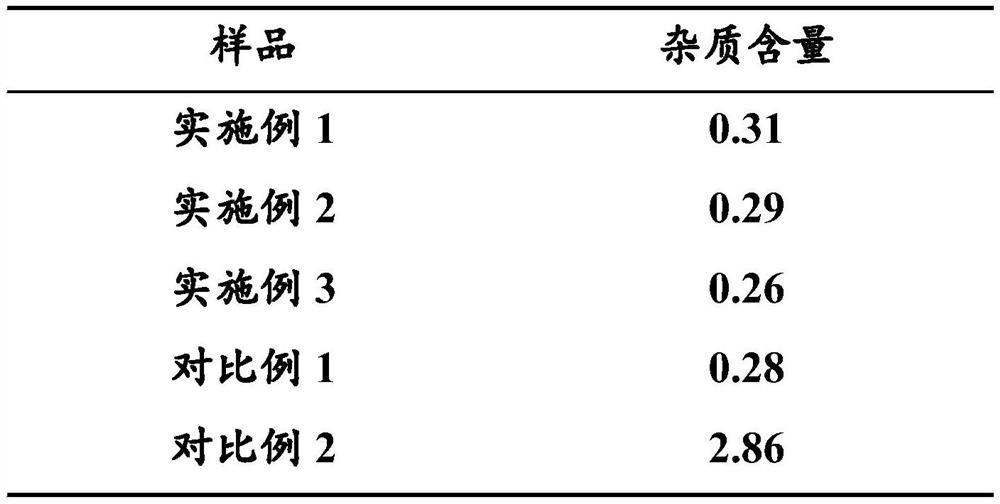
PendingCN112047991AResolution cycleSolution conditionsSteroidsDEXAMETHASONE SODIUM PHOSPHATE INJECTIONSodium phosphates
The invention belongs to the technical field of chemical engineering, and particularly relates to a method for efficiently removing dexamethasone sodium phosphate crystal water. According to the method, the crystal water in the dexamethasone sodium phosphate sample is efficiently removed by adopting a drying mode of vacuum sectional heating temperature control so that the method has the advantagesof short heating time, low heating temperature, high crystal water removal rate, no need of turning over the sample and the like; the problems that in the prior art, the operation period is long, conditions are not mild, products are prone to denaturation, operation steps are tedious and the like when dexamethasone sodium phosphate is dried to remove crystal water are solved. An injection preparation prepared from dexamethasone sodium phosphate without crystal water obtained by the method does not have white points and white blocks in the transportation and storage processes, is good in product quality stability, and has important significance for improving the yield and the finished product quality of the dexamethasone sodium phosphate injection.
Owner:西安国康瑞金制药有限公司
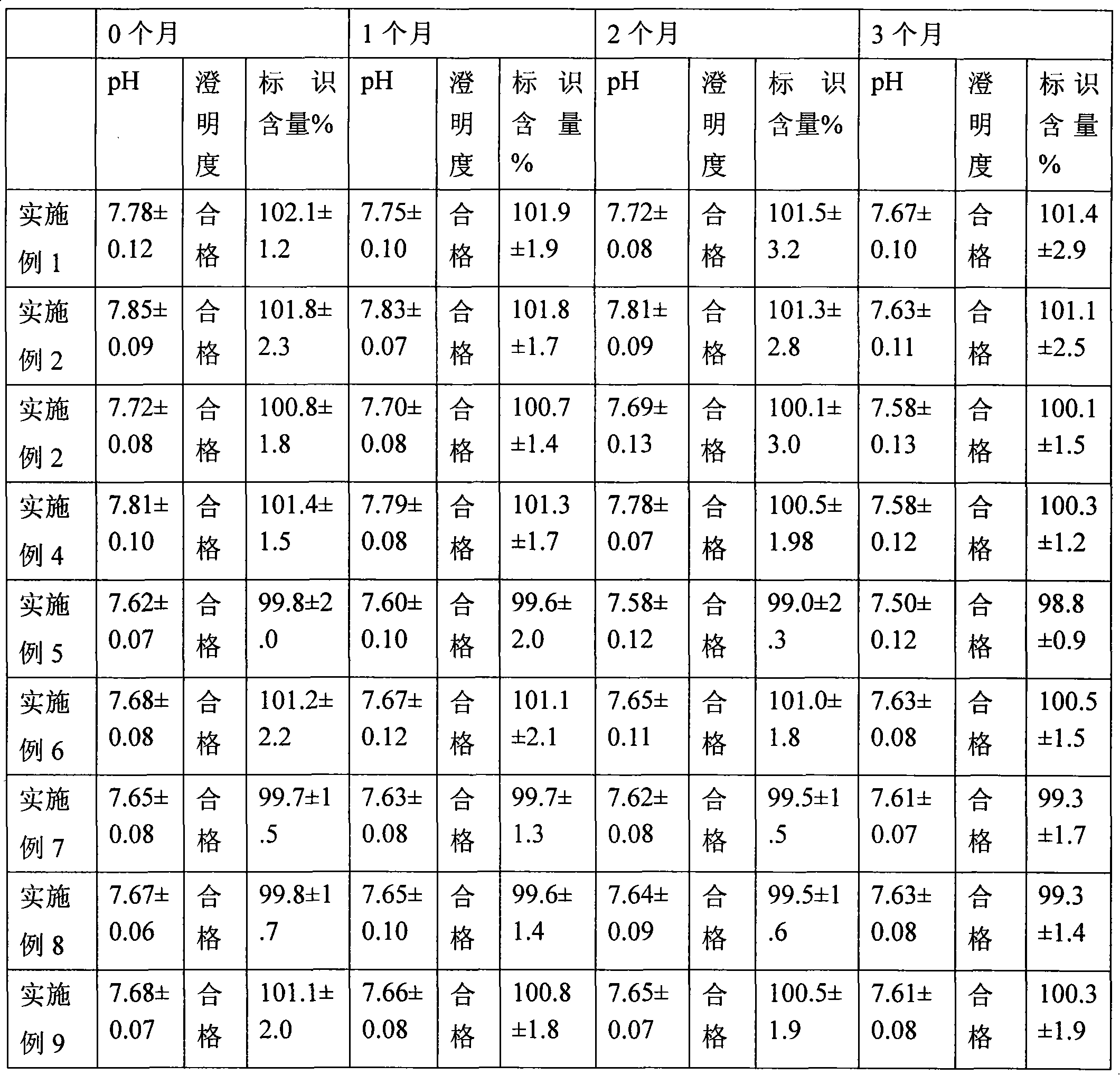
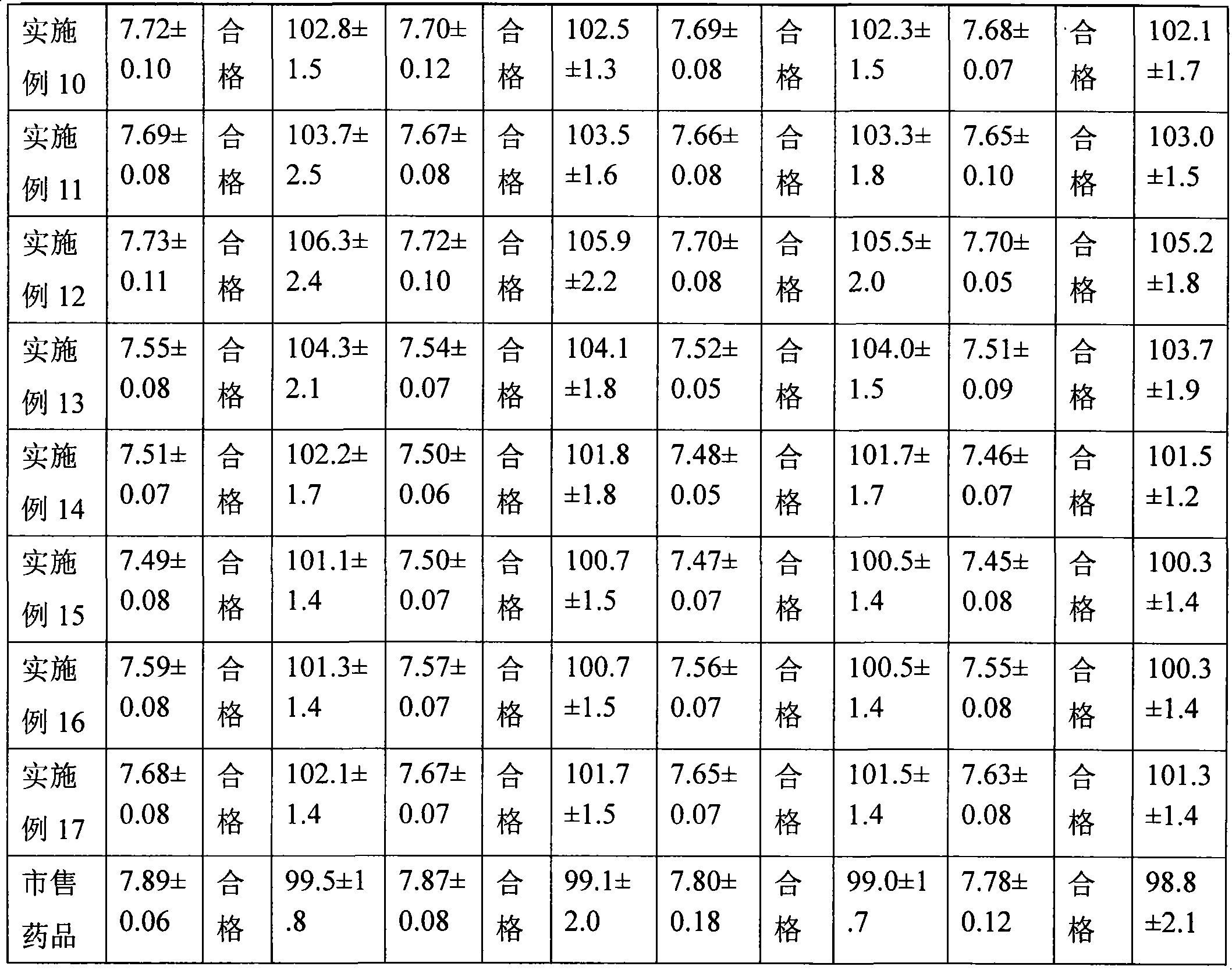
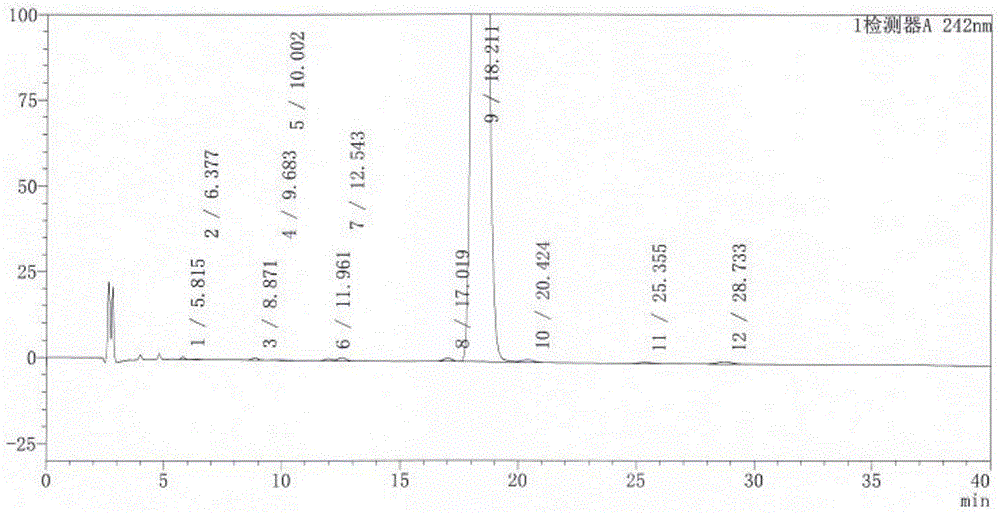
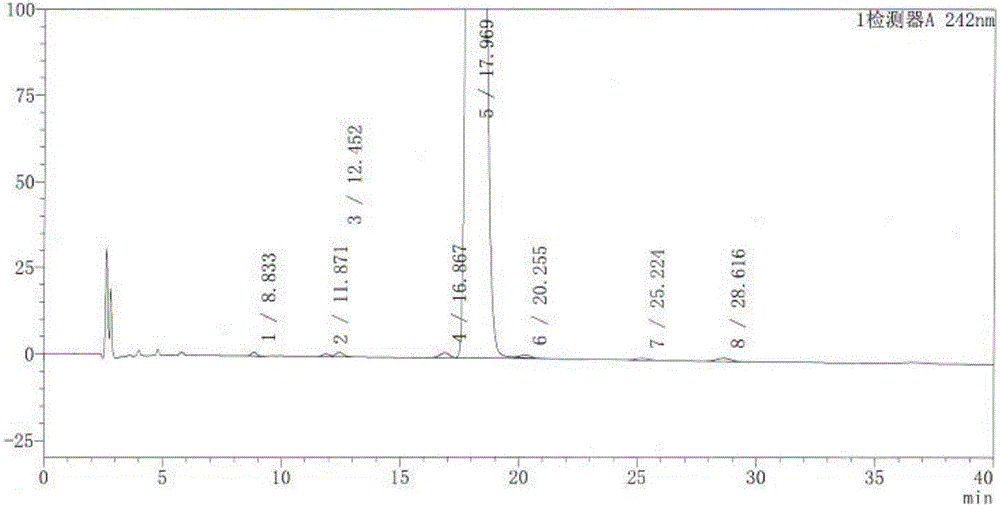
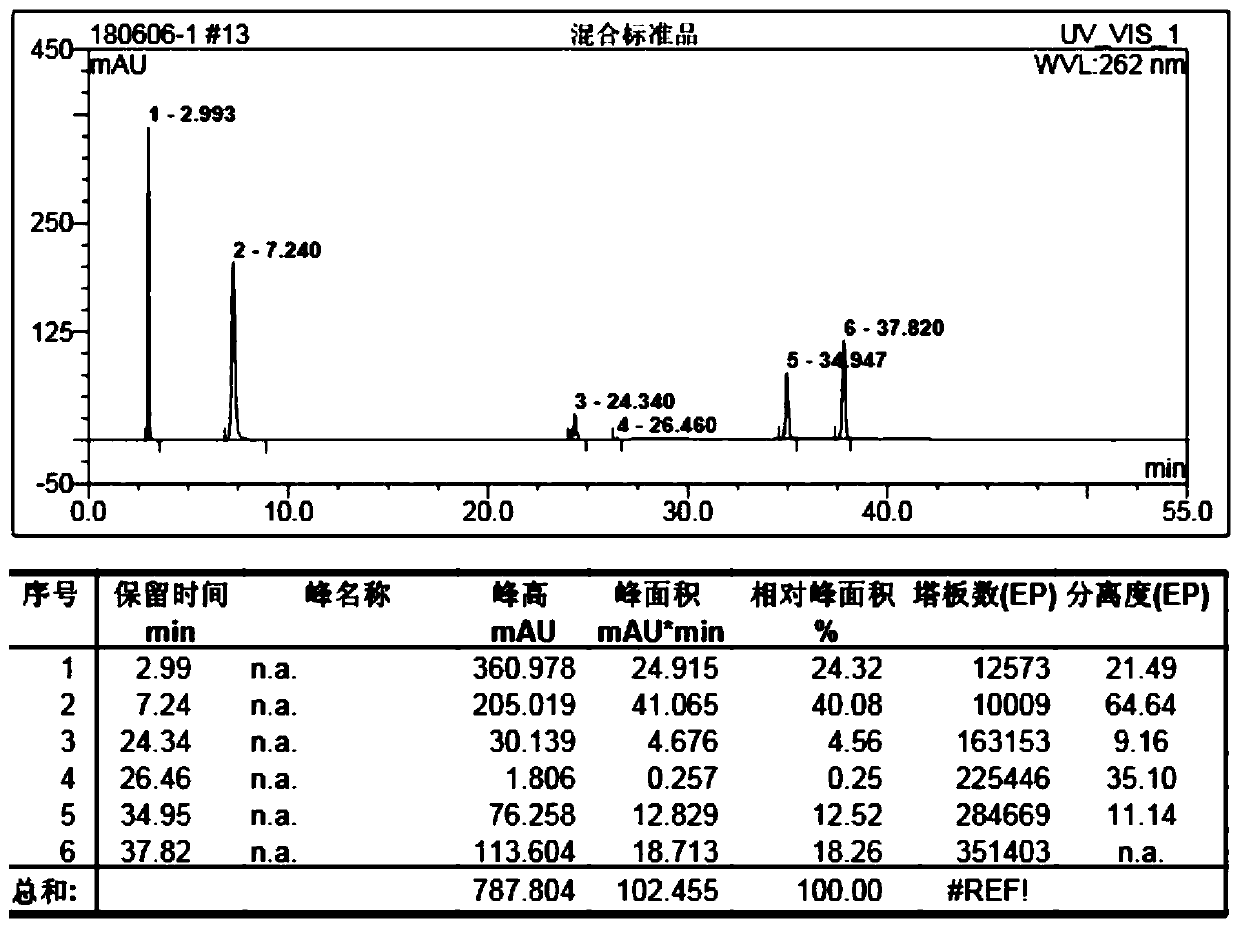
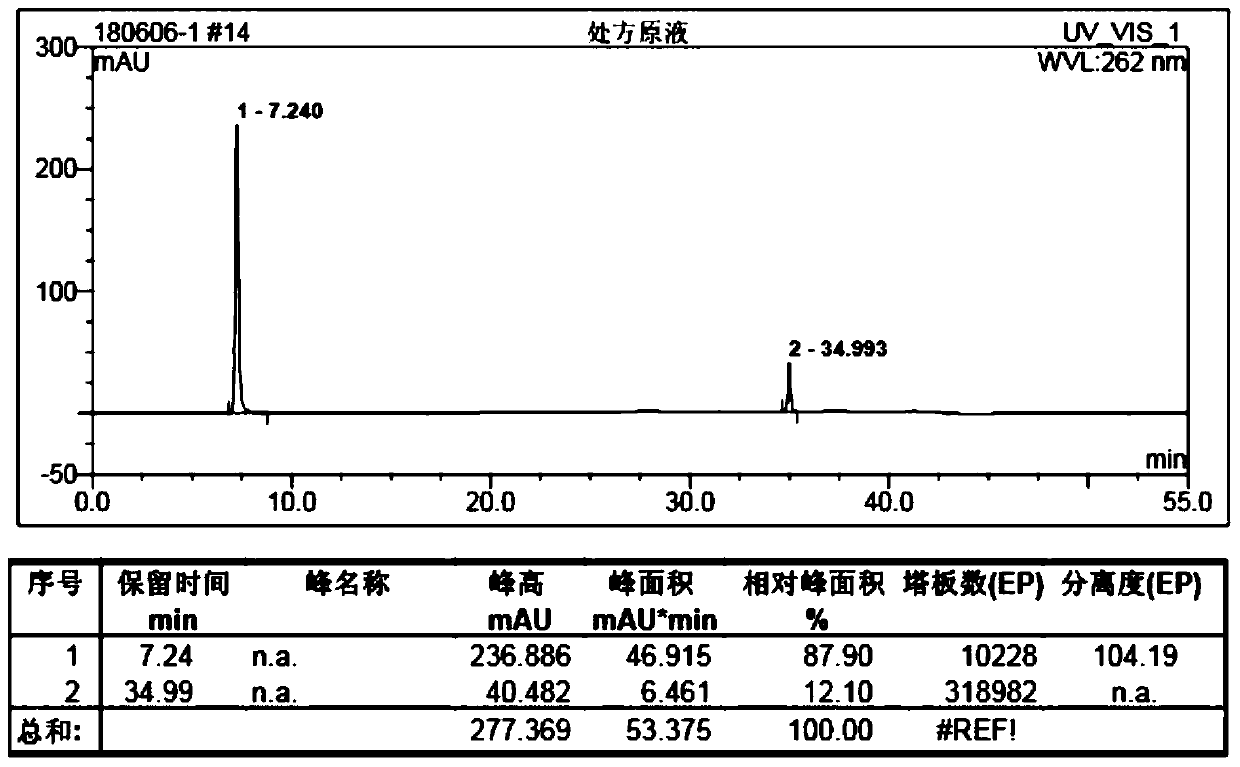
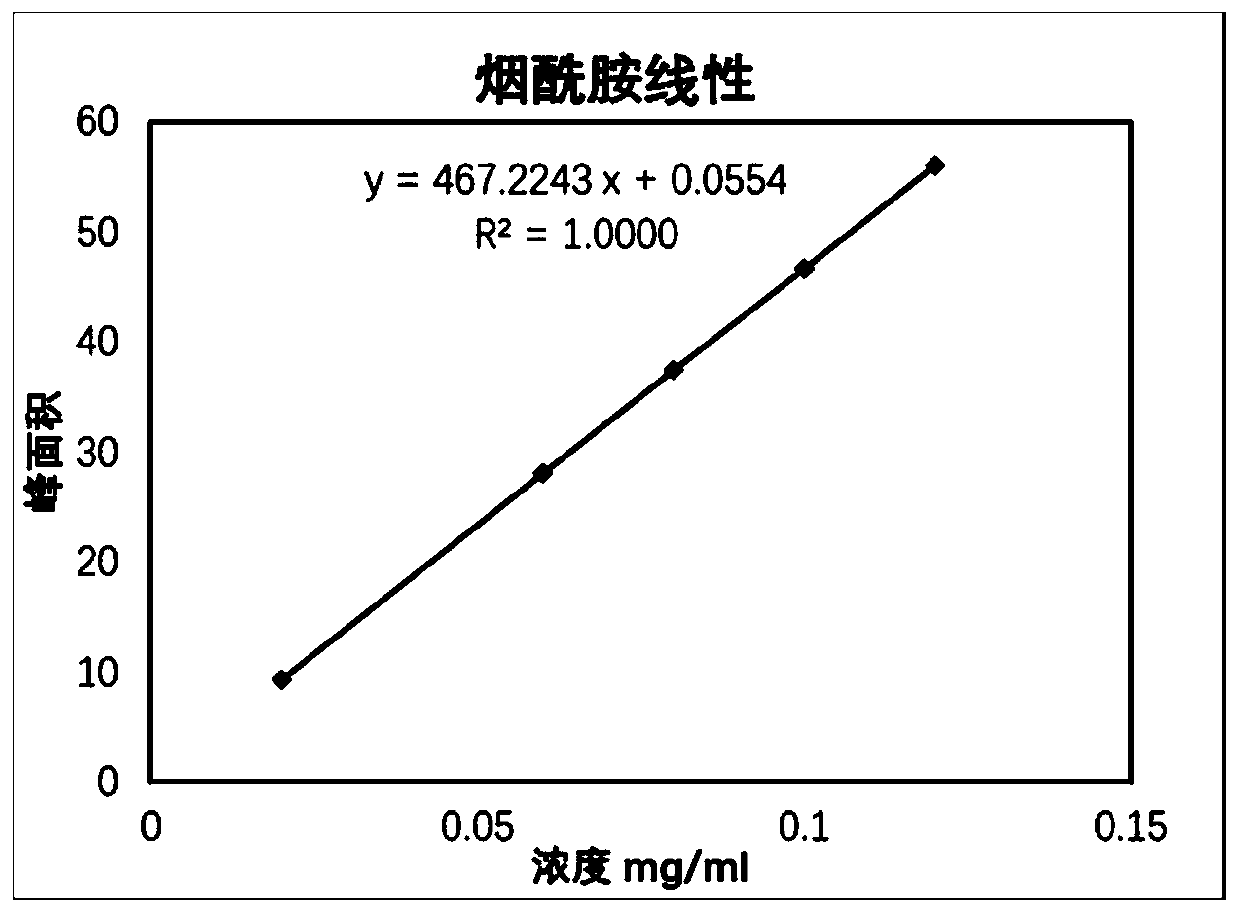
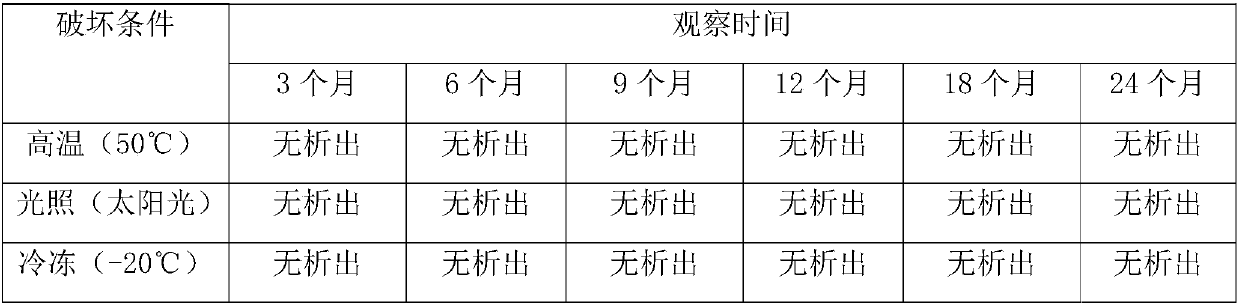
Quality standard of dexamethasone sodium phosphate injection and detection method thereof
The invention provides a quality standard detection method of a dexamethasone sodium phosphate injection. The quality standard detection method comprises the steps of: preparing a reference substancesolution, namely, weighing a nicotinamide reference substance, and adding water to dissolve the nicotinamide reference substance to prepare a nicotinamide reference substance solution; preparing a test sample solution, namely, measuring the dexamethasone sodium phosphate injection to be detected, and adding water to dissolve the dexamethasone sodium phosphate injection to prepare a nicotinamide test sample solution; preparing a system suitability solution, namely, measuring the dexamethasone sodium phosphate injection, and adding water to dilute the dexamethasone sodium phosphate injection toprepare a solution A; weighing a nicotinic acid reference substance, and adding water to dissolve the nicotinic acid reference substance to prepare a solution B; respectively measuring the solution Aand the solution B, and adding water to dilute the solution A and the solution B, so as to prepare a mixed solution containing nicotinic acid and nicotinamide; and injecting the prepared mixed solution into a liquid chromatograph for detection, respectively weighing the nicotinamide reference substance solution and the nicotinamide test sample solution and injecting them into the liquid chromatograph for detection if a separation degree of nicotinamide and nicotinic acid is not less than 3.0 and a number of theoretical plates calculated according to nicotinamide is not less than 2000, so as toobtain content of nicotinamide in the nicotinamide test sample solution.
Owner:武汉久安药业有限公司
Preparation method of dexamethasone sodium phosphate injection
InactiveCN105342992ASimple stepsRaw materials are easy to getOrganic active ingredientsAntipyreticDexamethasone acetatePhosphate
The invention relates to a preparation method of dexamethasone sodium phosphate injection. The preparation method comprises the following steps: synthetizing dexamethasone sodium phosphate; sequentially carrying out ring-opening reaction, recrystallization, base-catalyzed hydrolysis, pyrophosphoryl chloride esterification and salt-formation from neutralization to obtain a dexamethasone sodium phosphate solution, and carrying out further recrystallization on the dexamethasone sodium phosphate solution to obtain a dexamethasone sodium phosphate crystal by taking dexamethasone acetate epoxide as a starting material; drying the dexamethasone sodium phosphate crystal, and ball-milling the dried crystal into powder; and dissolving the dexamethasone sodium phosphate powder by water for injection for dilution, adding medicinal propylene glycol, and phosphate buffered solution with the pH being 8.0 to prepare the dexamethasone sodium phosphate injection. With the adoption of the method, the phosphate buffered solution is added into the dexamethasone sodium phosphate injection, so that the problem that the active ingredients of the dexamethasone sodium phosphate injection are decreased in efficacy is solved.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Dexamethasone sodium phosphate injection and preparation method thereof
InactiveCN109602701AStable pHExtended storage timeOrganic active ingredientsAntipyreticSulfite saltDisodium Edetate
The invention discloses a dexamethasone sodium phosphate injection and a preparation method thereof. The injection of the present invention is prepared from the following raw materials by weight percentages: 0.1-0.5% of dexamethasone sodium phosphate, 2.2-3.5% of propylene glycol, 0.03-0.08% of ethylenediaminetetraacetic acid disodium salt, 0.6-0.9% of disodium hydrogen phosphate, 0.1%-0.3% of anhydrous sodium sulfite, and the balance of water for injection. The invention also discloses a preparation method of the injection. The dexamethasone sodium phosphate injection prepared by the invention has stable pH value and long storage time, and all the indexes are qualified during a product validity period; and the method adopts the ethylenediaminetetraacetic acid disodium salt to effectivelyresist oxidation, and adopts hot red water for leak detection to effectively prevent crystals from being quenched by the liquid.
Owner:湖北久安医药集团有限公司
Skin disease medicine composition, preparation method and application thereof
InactiveCN106580929APlay a synergistic roleGood effectHeavy metal active ingredientsAntimycoticsDiseaseAlcohol
The invention provides a skin disease medicine composition. The composition is prepared from an alcohol-water solution with concentration exceeding 90%, fluconazole and sodium chloride injection and dexamethasone sodium phosphate injection in a volume ratio of 50:3-7:0.5-4. The chlorine content in the fluconazole and sodium chloride injection is 8.5-9.5g / L, and the content of the fluconazol is 1.5-2.5g / L. The type of the dexamethasone sodium phosphate injection is 1ml:1mg, 1ml:2mg or 1ml:5mg. A method and an application of the skin disease medicine composition are further provided. Preferably the diseases include mycosis ungualis, tinea of feet and hands, itching skin and pruritus of perineum.
Owner:贺敏
Freeze-dried powder injection containing dexamethasone sodium phosphate
ActiveCN111773187AAvoid damageImprove securityPowder deliveryOrganic active ingredientsSodium phosphatesPharmaceutical Aids
The invention belongs to the field of biomedicine, and relates to a freeze-dried powder injection containing a dexamethasone medicine. The freeze-dried powder injection preparation provided by the present invention is a dexamethasone-type drug freeze-dried powder injection preparation without a freeze-dried support agent or excipient or stent. By tympanum administration, on one hand, the freeze-dried powder injection preparation can reduce the whole-body toxic and side effects of oral administration and play a role in lesion parts; on the other hand, the preparation can effectively prevent hydrolysis of the medicine and improve the bioavailability of the medicine compared with common aqueous injections. The freeze-dried powder injection does not require a stent, and at the same time has amore suitable osmotic pressure than commercially available dexamethasone sodium phosphate injection solutions, avoids the deposition of excessive auxiliary materials in the middle ear and the damage of hypertonic fluid to local tissues, and can increase the safety of tympanum administration. In addition, the prepared freeze-dried powder preparation has fewer types and small dosages of auxiliary materials, reduces the interaction between medicines, and has good safety for industrial production and clinical medication.
Owner:和舆(苏州)医药科技有限公司
A kind of dexamethasone sodium phosphate injection
The invention relates to a dexamethasone sodium phosphate injection and a preparation method thereof. The injection is prepared from dexamethasone sodium phosphate, a stabilizer, an antioxidant, a metal ion chelant, a pH regulator, a viscosity regulator, a solubilizer, an osmotic pressure regulator and water for injection by dissolving and high-temperature sterilization, and has certain viscosity.The dexamethasone sodium phosphate injection is applied to intratympanic injection, and has the effects of enhancing intratympanic medicine absorption and improving sudden deafness.
Owner:和舆(苏州)医药科技有限公司
Preparation technology of dexamethasone sodium phosphate injection
InactiveCN105342993ASimple stepsRaw materials are easy to getOrganic active ingredientsAntipyreticDexamethasone acetatePhosphate
The invention relates to a preparation technology of dexamethasone sodium phosphate injection. The preparation technology comprises the following steps: synthetizing dexamethasone sodium phosphate; sequentially carrying out ring-opening reaction, recrystallization, base-catalyzed hydrolysis, pyrophosphoryl chloride esterification and salt-formation from neutralization to obtain a dexamethasone sodium phosphate solution, and carrying out further recrystallization on the dexamethasone sodium phosphate solution to obtain a dexamethasone sodium phosphate crystal by taking dexamethasone acetate epoxide as a starting material; drying the dexamethasone sodium phosphate crystal, and ball-milling the dried crystal into powder; and dissolving the dexamethasone sodium phosphate powder by water for injection for dilution, adding medicinal propylene glycol, and phosphate buffered solution with the pH being 8.0 to prepare the dexamethasone sodium phosphate injection. With the adoption of the method, the problems that in the existing preparation methods, the yield is low, and the active ingredients of the prepared dexamethasone sodium phosphate injection are rapid in efficacy-decreasing speed are solved.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Dexamethasone sodium phosphate injection and preparation method thereof
ActiveCN113730348AEnsure safetyAdequate safety dataOrganic active ingredientsPharmaceutical delivery mechanismDisodium EdetateSodium phosphates
The invention discloses a dexamethasone sodium phosphate injection and a preparation method thereof and belongs to the technical field of pharmaceutical preparations. The dexamethasone sodium phosphate injection comprises the following components in percentage by weight: 0.2-0.5% of dexamethasone sodium phosphate, 1.0-2.0% of xylitol, 0.5-1.0% of glycerol, 0.005-0.01% of disodium edetate and the balance of water. The dexamethasone sodium phosphate injection provided by the invention has an unexpected stability advantage, the occurrence of a hydrolysis reaction can be effectively controlled, and the content of a hydrolyzed impurity dexamethasone is lowered; an antioxidant sodium hydrogen sulfite is not used in a prescription, so that the generation of sulfonated impurities is avoided; and on the other hand, a sterile guarantee method of sterilization and filtration is adopted, so that the hydrolysis reaction of the injection caused by a high-temperature process is further avoided.
Owner:HENAN RUNHONG PHARMA
Pharmaceutical composition containing tropisetron hydrochloride and dexamethasone sodium phosphate
InactiveCN103393701BImprove antiemetic efficacyDigestive systemHeterocyclic compound active ingredientsFructoseRinger's solution
Owner:HAINAN LINGKANG PHARMA CO LTD
Intravenous injection medication method for cardia-cerebrovascular diseases
InactiveCN108452170ASimple treatmentGood effectOrganic active ingredientsNervous disorderRegimenVitamin C
The invention discloses an intravenous injection medication method for cardia-cerebrovascular diseases. The compatibility of medicines comprises pulse-activating powder, radix astragali, flos carthami, a Qingkailing compound, tetramethylprazine, radix salviae miltiorrhizae, puerarin, vitamin C and a dexamethasone sodium phosphate injection; all preparations are proportioned according to the stipulation of the specification, and the injection consists of pulse-activating powder, radix astragali, flos carthami, the Qingkailing compound, tetramethylprazine, radix salviae miltiorrhizae, puerarin,vitamin C, dexamethasone sodium phosphate, 0.9% sodium chloride and a 5% glucose injection. The using method comprises the following steps: taking the preparations to be dissolved into 250 ml or 500 ml of 0.9% sodium chloride or 5% glucose injection respectively every day, and carrying out intravenous drip, once a day for 15 days as a course of treatment. The intravenous injection medication method is simple in operation, high in cure rate, low in price, convenient for material drawing and free of risk, and also has the effects of effectively curing and dilating coronary artery and blood vessels of brain, increasing coronary flow, reducing cardiac and cerebral oxygen consumption, reducing blood viscosity, resisting platelet aggregation, improving microcirculation and enhancing immunologicfunction; and moreover, the combination of Chinese traditional and Western medicine also highlights the characteristics of Chinese traditional medicine.
Owner:陕西玖易大药房有限公司
A kind of dexamethasone sodium phosphate freeze-dried powder injection
The invention belongs to the field of biomedicine and relates to a freeze-dried powder injection containing dexamethasone. The freeze-dried powder injection preparation provided by the present invention is a freeze-dried powder injection preparation of dexamethasone drugs without freeze-dry support agent or excipient or scaffolding agent. Administration of the freeze-dried powder preparation through the tympanic cavity, on the one hand, can reduce the systemic toxic and side effects of oral administration, and play a role in the lesion; on the other hand, compared with ordinary water injection, the preparation can effectively prevent drug hydrolysis and improve the biological activity of the drug Utilization. The freeze-dried powder injection involved in the present invention does not require a scaffolding agent, and has a more suitable osmotic pressure than the commercially available dexamethasone sodium phosphate injection, and avoids excessive deposition of auxiliary materials in the middle ear and damage to local tissues caused by hypertonic fluid. Can increase the safety of intratympanic administration. In addition, the freeze-dried powder preparation prepared by the invention has fewer types and dosages of auxiliary materials, reduces the interaction between drugs, and has good safety for industrial production and clinical medication.
Owner:HEYU (SUZHOU) PHARM TECH CO LTD
Dexamethasone sodium phosphate injection
ActiveCN101120915BImprove product qualityReduce adverse reactionsOrganic active ingredientsAntipyreticPhosphatePropanediol
Dexamethasone sodium phosphate injection comprises the medicinal propanediol with the volume ratio of 4 percent to 6 percent, the phosphate buffer of 0.3 percent to 0.5 percent with the PH value of 8.0, the dexamethasone sodium phosphate and the injection water. Content of the dexamethasone sodium phosphate is 2mg to 5 mg per ml. The application is safe and the cost is low in the present invention.
Owner:TIANJIN PHARMA GROUP XINZHENG
Nasal drip liquor for treating rhinitis and nasosinusitis
InactiveCN100415244CSignificant effectSymptoms improvedOrganic active ingredientsPharmaceutical delivery mechanismSinusitisTherapeutic effect
A kind of nasal drops for treating rhinitis and nasosinusitis is prepared from chloromycetin injection, dexamethasone-sodium phosphate injection, chlorphenamine maleate injection, ribavirin injection, metronidazole injection and lidocaine hydrochloride injection.
Owner:刘明芳
Dexamethasone sodium phosphate freeze-dried powder injection
Owner:和舆(苏州)医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com