Quality standard of dexamethasone sodium phosphate injection and detection method thereof
A technology of dexamethasone sodium phosphate and quality standards, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of not being able to represent product quality well, and achieve the effect of improving control and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] In order to make the purpose, technical solution and advantages of the present invention clearer, the embodiments of the present invention will be further described below in conjunction with the accompanying drawings.
[0020] In the following examples:
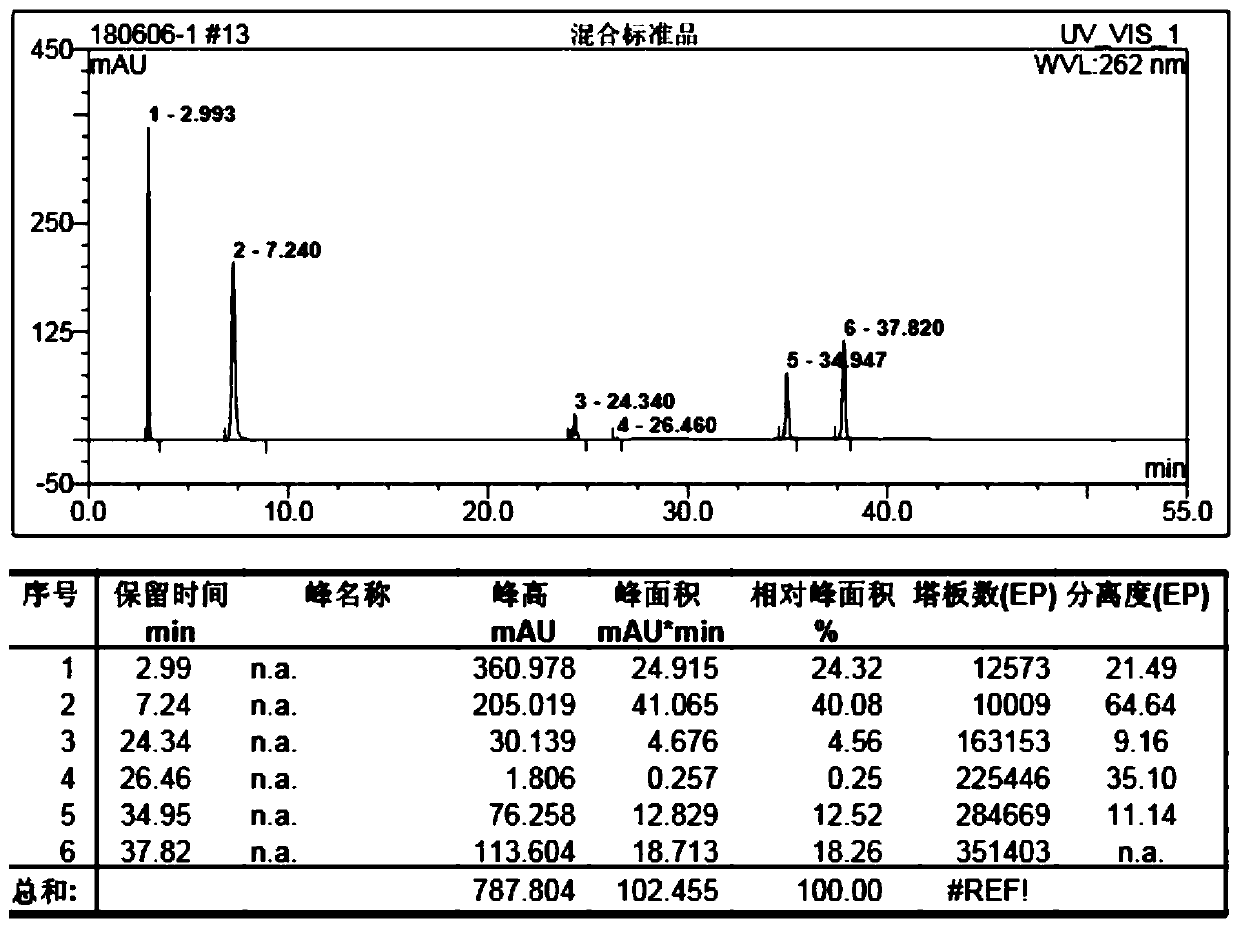
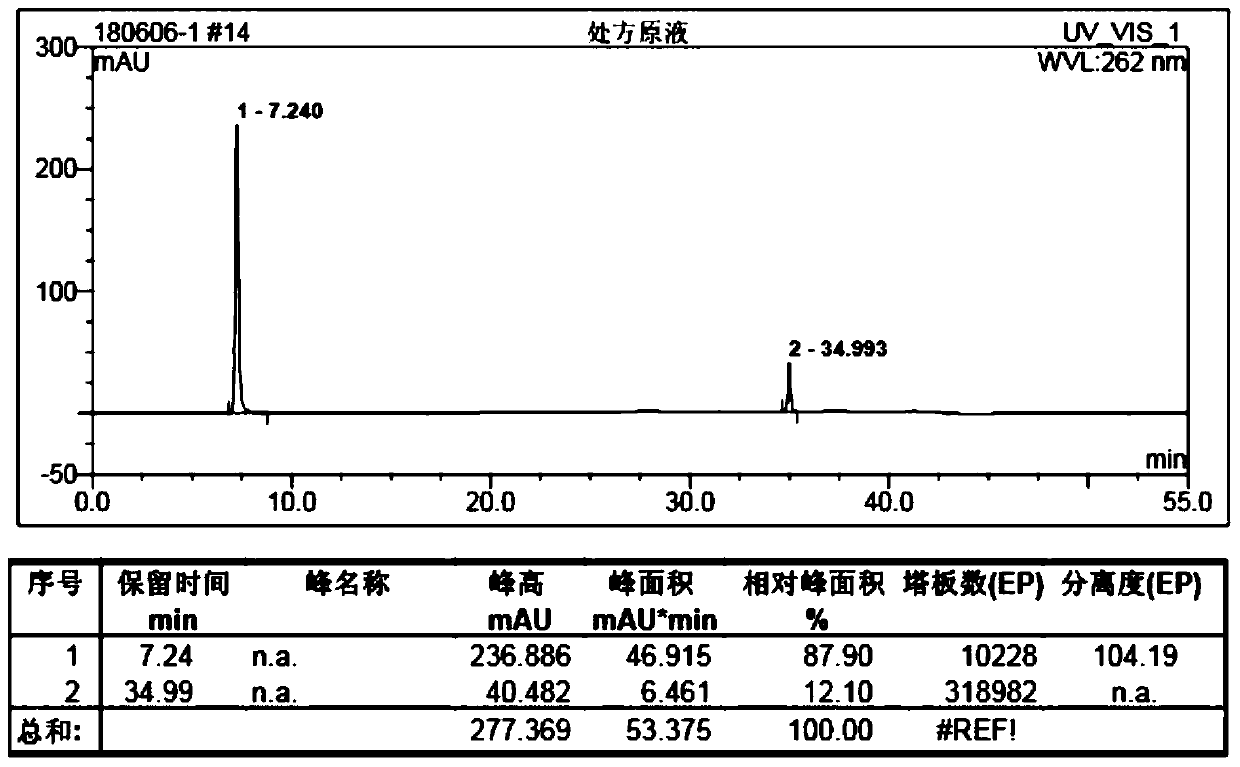
[0021] The setting conditions of the liquid chromatograph are: use octadecylsilane bonded silica gel as filler; use methanol-0.005M (M represents mol / l) sodium heptanesulfonate solution as mobile phase, methanol and heptanesulfonic acid The volume ratio of the sodium solution is 30:70; the detection wavelength is 262nm; the flow rate is 1.0ml / min; the column temperature is 30°C.
[0022] Instruments and reagents: Diana U-3000 high performance liquid chromatography; American Agilent high performance liquid chromatography; VWD variable wavelength ultraviolet detector; intelligent column thermostat; column: Thermo C18 150×4.6mm.
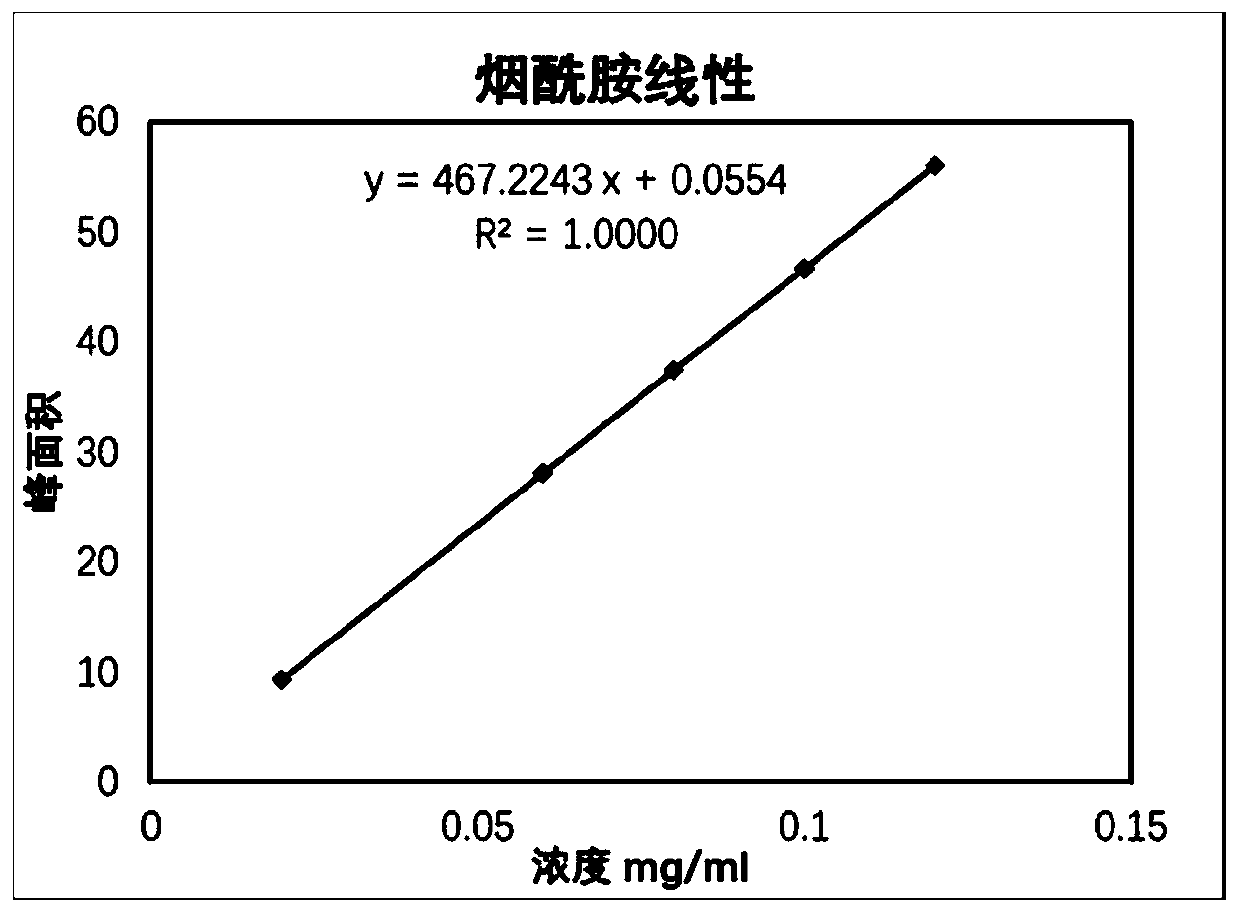
[0023] Niacinamide reference substance: purchased from the China Inspection Institute; niac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com