High-safety dexamethasone sodium phosphate injection and preparation technology thereof
A dexamethasone sodium phosphate, high-safety technology, applied in the field of pharmacy, can solve problems such as blindness, corneal damage, allergic reactions, etc., and achieve the effects of ensuring stability, controlling the generation of impurities, and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
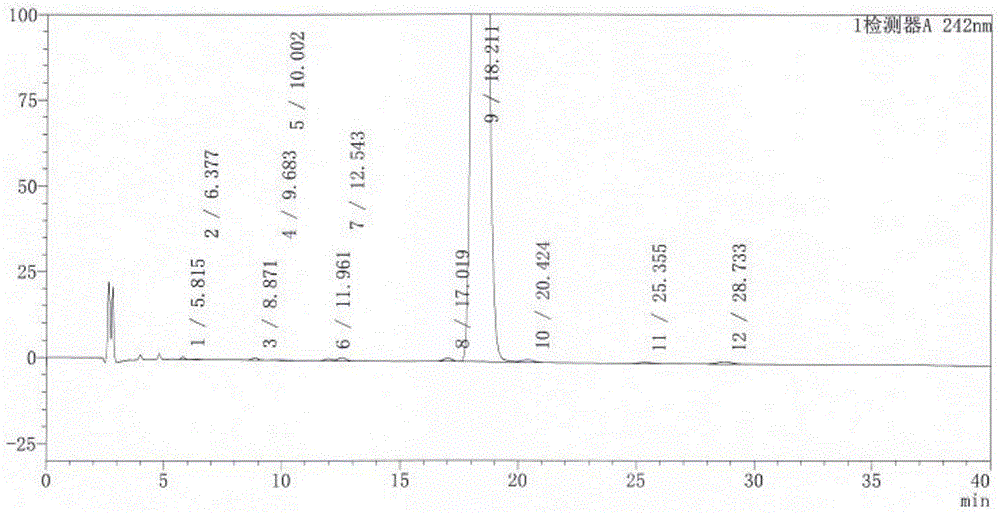
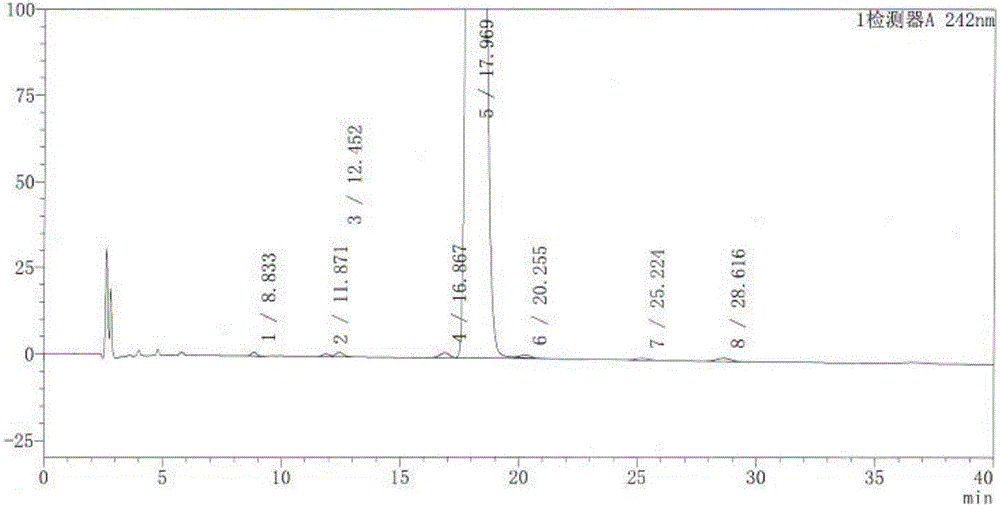
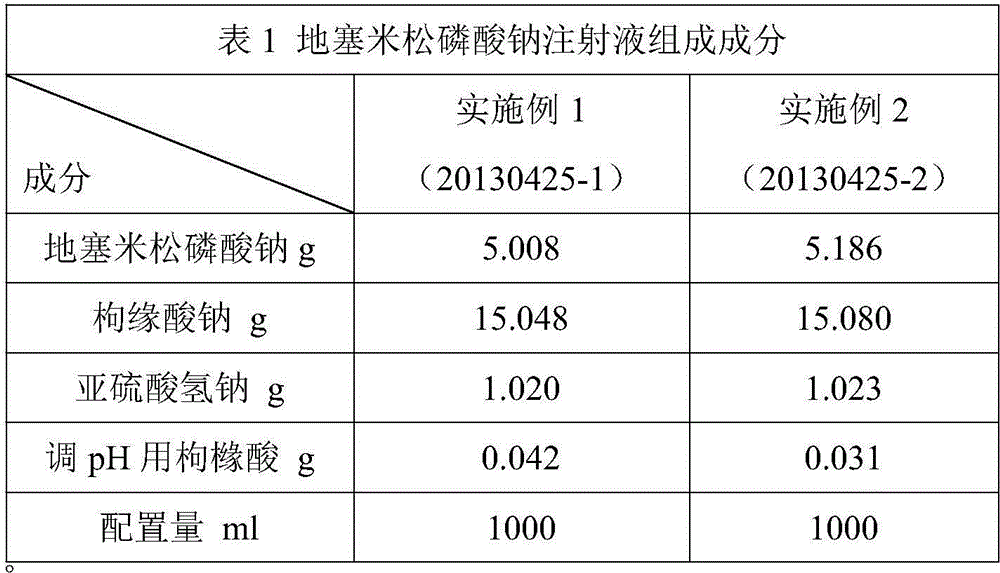
[0027] "Chinese Pharmacopoeia" for dexamethasone sodium phosphate injection:
[0028] "Chinese Pharmacopoeia" (2010 Edition, Part II) stipulates that this product is a sterile aqueous solution of dexamethasone sodium phosphate, and the content of dexamethasone sodium phosphate should be 90.0% to 110.0% of the labeled amount. Regulations for related substances: if there is a peak with the same retention time as that of dexamethasone in the chromatogram of the reference substance solution in the chromatogram of the test solution of dexamethasone sodium phosphate, it shall be calculated by the peak area according to the external standard method, and the marked amount shall not be exceeded If other impurity peaks are evident, the peak area of the impurity (impurity I) with a relative retention time of about 0.25 to the dexamethasone sodium phosphate peak shall not be greater than the main peak area (1.0%) of the contrast solution, and the peak area of other single impurities sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com