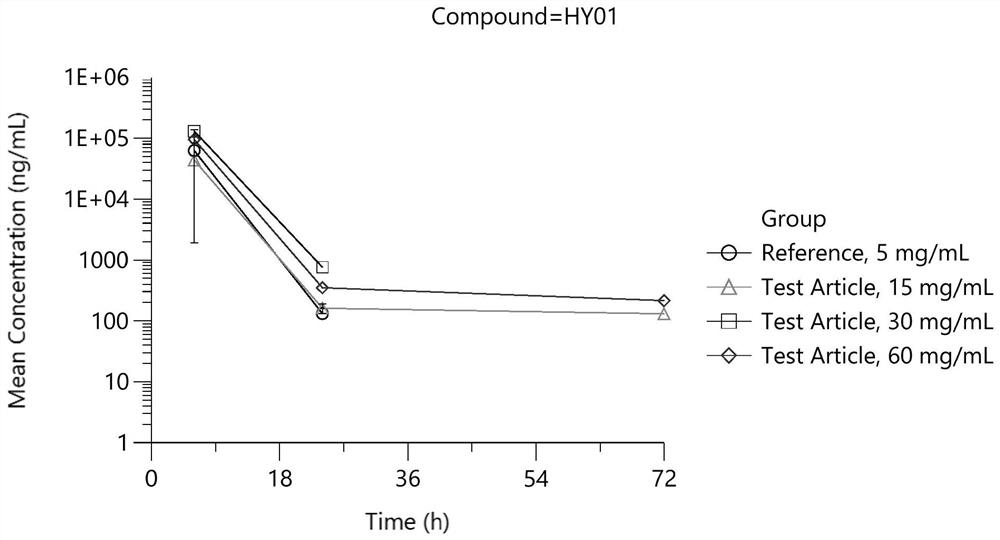
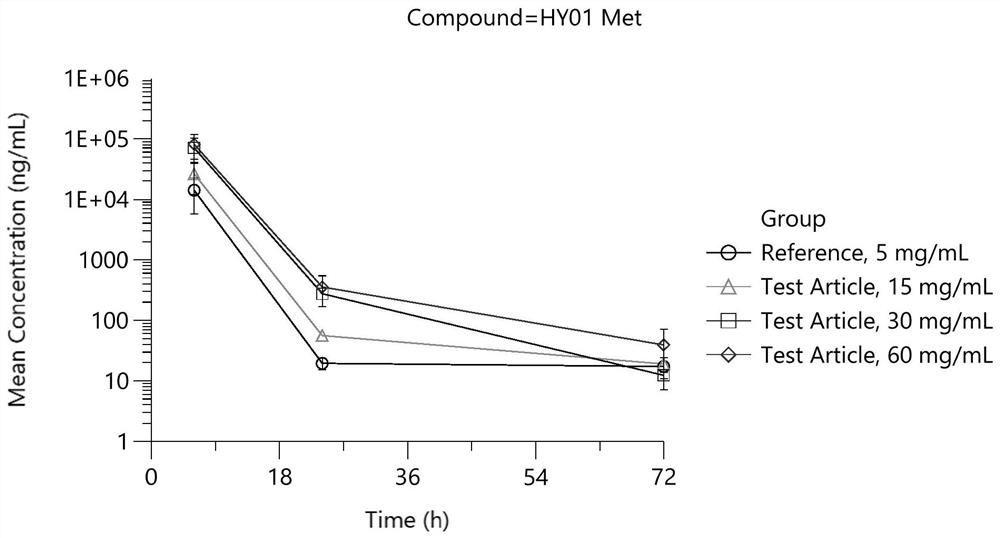
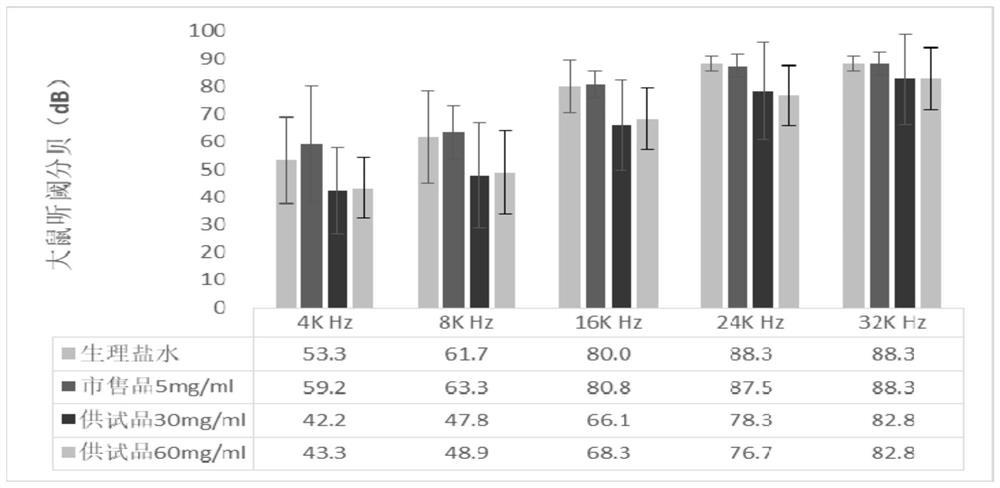
A kind of dexamethasone sodium phosphate freeze-dried powder injection
A technology of dexamethasone sodium phosphate and freeze-dried powder injection, applied in the field of biomedicine, can solve the problems of drug refractory concentration, obvious systemic side effects, etc., and achieve the effects of obvious curative effect, protection and promotion of recovery, and rapid progress.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] The formula and preparation method of embodiment 1 dexamethasone sodium phosphate injection
[0113] Table 2
[0114]
[0115] Preparation:
[0116] (1) dissolving disodium hydrogen phosphate and sodium dihydrogen phosphate of prescription quantity into phosphate buffer saline solution of pH8.0 with water for injection cooled to room temperature of 90% of prescription quantity;
[0117] (2) Add the dexamethasone sodium phosphate and EDTA-2NaCa of the prescription amount to the solution in (1) and stir to dissolve, measure the pH, and adjust the pH to 8.0±.005;
[0118] (3) Dilute to a 50mL measuring bottle and shake well;
[0119] (4) Sterilize and filter the medicinal solution, pack in 3mL vials, 1mL per bottle, and use freeze-drying technology to make the medicinal solution into sterile powder, to obtain final product;
[0120] (5) Gained sterile powder is a white loose lump;
[0121] (6) The temperature is controlled at room temperature throughout the preparat...
Embodiment 2
[0122] The formula and preparation method of embodiment 2 dexamethasone sodium phosphate injection
[0123] table 3
[0124]
[0125] Preparation:
[0126] (1) the water for injection is cooled to room temperature;
[0127] (2) Dissolve the prescribed amount of dexamethasone sodium phosphate and EDTA-2NaCa with the above-mentioned water for injection, and adjust the pH to 8.0 ± 0.05 with a pH regulator;
[0128] (3) Dilute to full volume and shake well;
[0129] (4) The medicinal liquid is sterilized and filtered, and the medicinal liquid is made into sterile powder by using freeze-drying technology, and then packed in 3mL vials, 1mL per bottle, to obtain the product;
[0130] (5) Gained sterile powder is a white loose lump;
[0131] (6) The temperature is controlled at room temperature throughout the preparation process.
Embodiment 3
[0132] The formula and preparation method of embodiment 3 dexamethasone sodium phosphate injection
[0133] Table 4
[0134]
[0135] Preparation:
[0136] (1) Dissolving the dexamethasone sodium phosphate and auxiliary materials of the prescription amount with water for injection at room temperature;
[0137] (2) Adjust the pH of the medicinal solution to 8.0±0.05 with 0.1M HCl;
[0138] (3) Sterile filtration, subpackage in 3mL vials, 1mL per bottle, apply freeze-drying technology to make the medicinal liquid into sterile powder, to get final product;
[0139] (4) Gained sterile powder is a white loose lump;
[0140] (5) The temperature is controlled at room temperature throughout the preparation process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com