Skin disease medicine composition, preparation method and application thereof
A composition and skin disease technology, applied in the direction of drug combination, skin disease, pharmaceutical formula, etc., can solve the problems of long treatment time, poor curative effect, easy recurrence, etc., and achieve the effect of simple preparation method, good curative effect, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
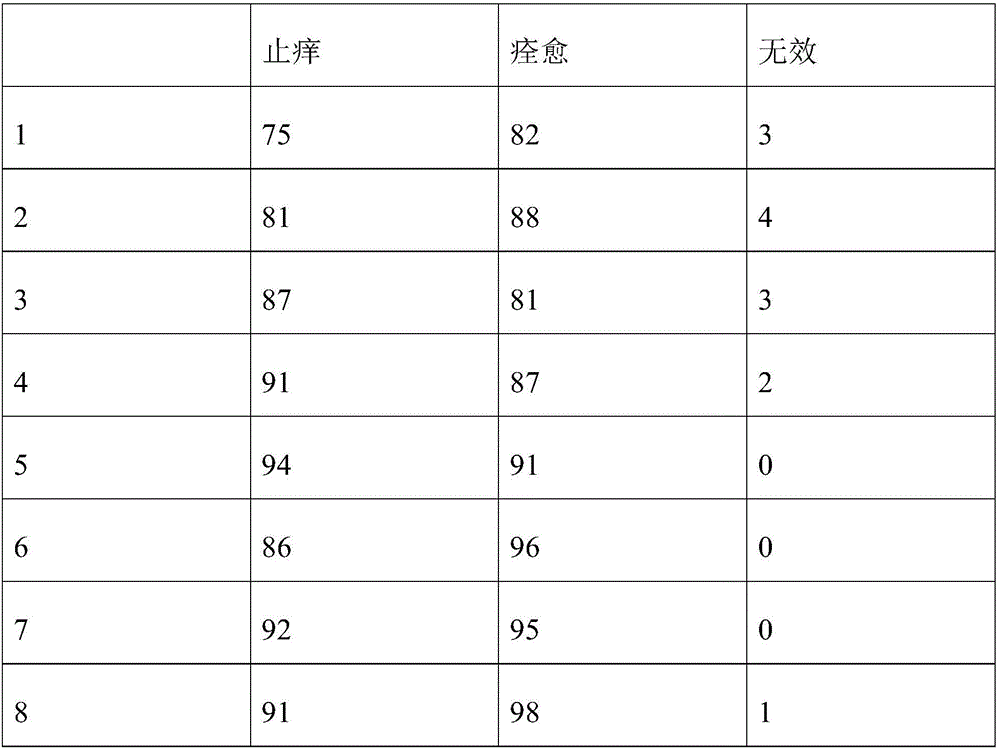
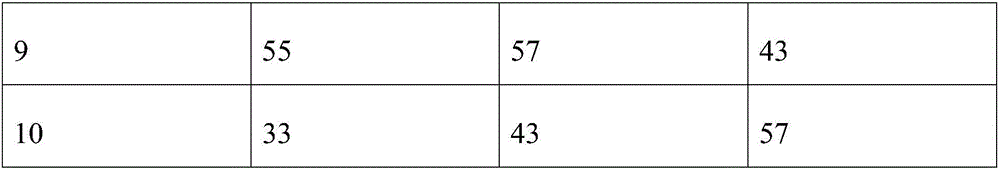
Examples
Embodiment 1
[0031] Formulating Dermatological Sprays
[0032] The formula is as follows:
[0033] 90% alcohol 50ml
[0034] Fluconazole sodium chloride injection (sodium chloride content 8.5g / L, fluconazole content 1.5g / L) 7ml
[0035] Dexamethasone sodium phosphate injection (1ml: 1mg) 4ml.
[0036] The above three solutions were mixed evenly, stirred at an ambient temperature of 15° C. for 10 min, and then left to stand at a room temperature of 25° C. The standing time is 12h. Afterwards, the mixed liquid medicine is obtained for spraying.
Embodiment 2
[0038] Formulating Dermatological Sprays
[0039] The formula is as follows:
[0040] 95% alcohol 50ml
[0041] Fluconazole sodium chloride injection (sodium chloride content 9.5g / L, fluconazole content 1.5g / L) 3ml
[0042] Dexamethasone sodium phosphate injection (1ml: 5mg) 0.5ml.
[0043] Mix the above three solutions evenly, stir for 10 min at an ambient temperature of 20°C, and then stand still at a room temperature of 25°C. The standing time is 24h. Afterwards, the mixed liquid medicine is obtained for spraying.
Embodiment 3
[0045] Formulating Dermatological Sprays
[0046] The formula is as follows:
[0047] 95% alcohol 50ml
[0048] Fluconazole sodium chloride injection (sodium chloride content 9g / L, fluconazole content 1g / L) 4ml
[0049] Dexamethasone sodium phosphate injection (1ml: 2mg) 1ml.
[0050] The above three solutions were mixed evenly, stirred for 7 minutes at an ambient temperature of 20°C, and then left to stand at a room temperature of 25°C. The standing time is 20h. Afterwards, the mixed liquid medicine is obtained for spraying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com