Dexamethasone sodium phosphate injection and preparation method thereof
A technology of dexamethasone sodium phosphate and injection, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of user product scrapping and waste of resources, and achieve storage time Longevity, prevention of precipitation of crystals, and stable pH
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
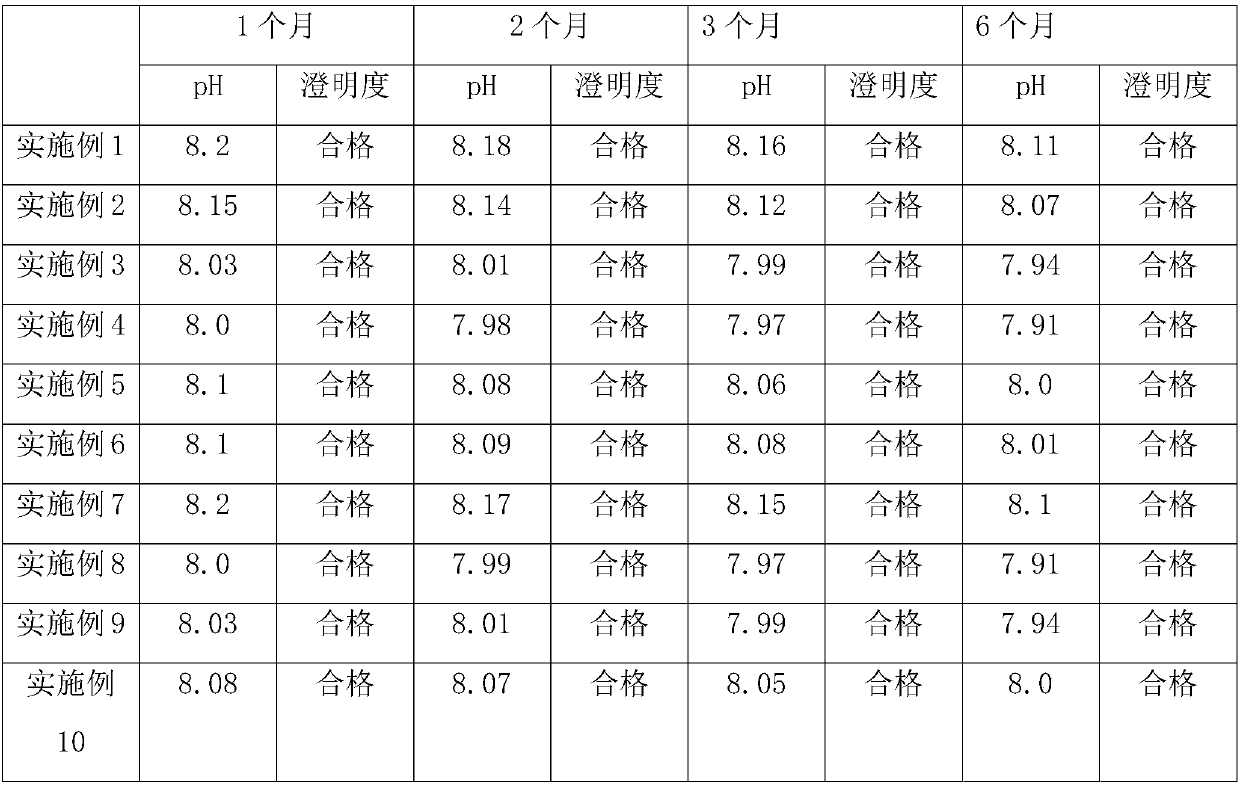
[0022] A dexamethasone sodium phosphate injection, which is made of the following raw materials: dexamethasone sodium phosphate 2g, propylene glycol 22g, edetate disodium 0.3g, disodium hydrogen phosphate 6g, anhydrous sodium sulfite 1g, injection Dilute to 1000ml with water.
[0023] The preparation method of the present invention is as follows:
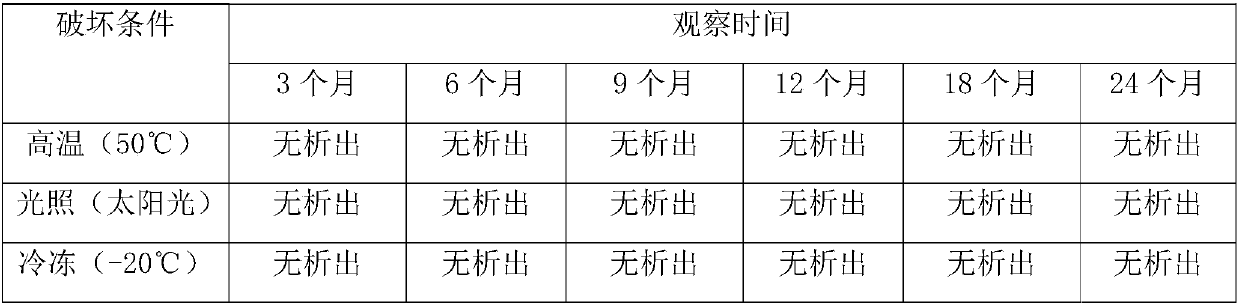
[0024] Take an appropriate amount of water for injection, add disodium hydrogen phosphate, stir to dissolve, add anhydrous sodium sulfite and disodium edetate, stir to dissolve, fill with nitrogen for 10 minutes, add dexamethasone sodium phosphate and propylene glycol, stir to dissolve, and then use 10% hydrogen Adjust the pH value to 8.0-8.2 with sodium oxide, add water for injection to 1000ml, then add 0.1% activated carbon, fill with nitrogen, let it stand for 20 minutes, decarbonize, fine filter with microporous membrane, potting, and sterilize with fluid steam at 100°C 20 minutes, and finally use 50 ℃ red water to check for le...
Embodiment 2
[0027] A dexamethasone sodium phosphate injection, which is made of the following raw materials: dexamethasone sodium phosphate 5g, propylene glycol 35g, edetate disodium 0.8g, disodium hydrogen phosphate 9g, anhydrous sodium sulfite 3g, injection Dilute to 1000ml with water.
[0028] The preparation method of the present invention is as follows:
[0029] Take an appropriate amount of water for injection, add disodium hydrogen phosphate, stir to dissolve, add anhydrous sodium sulfite and disodium edetate, stir to dissolve, fill with nitrogen for 10 minutes, add dexamethasone sodium phosphate and propylene glycol, stir to dissolve, and then use 10% hydrogen Adjust the pH value to 8.0-8.2 with sodium oxide, add water for injection to 1000ml, then add 0.1% activated carbon, fill with nitrogen, let it stand for 20 minutes, decarbonize, fine filter with microporous membrane, potting, and sterilize with fluid steam at 100°C 20 minutes, and finally use 50 ℃ red water to check for le...
Embodiment 3
[0032] A dexamethasone sodium phosphate injection, which is made of the following raw materials: dexamethasone sodium phosphate 2g, propylene glycol 25g, edetate disodium 0.4g, disodium hydrogen phosphate 6.5g, anhydrous sodium sulfite 1.5g , dilute to 1000ml with water for injection.
[0033] The preparation method of the present invention is as follows:
[0034] Take an appropriate amount of water for injection, add disodium hydrogen phosphate, stir to dissolve, add anhydrous sodium sulfite and disodium edetate, stir to dissolve, fill with nitrogen for 10 minutes, add dexamethasone sodium phosphate and propylene glycol, stir to dissolve, and then use 10% hydrogen Adjust the pH value to 8.0-8.2 with sodium oxide, add water for injection to 1000ml, then add 0.1% activated carbon, fill with nitrogen, let it stand for 20 minutes, decarbonize, fine filter with microporous membrane, potting, and sterilize with fluid steam at 100°C 20 minutes, and finally use 50 ℃ red water to che...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com