Preparation method of compound serving as neuroprotective agent
A compound and hydroxyl technology, applied in the field of compound preparation, can solve the problems of difficult large-scale industrial production, low yield, and many steps, and achieve the effect of alleviating Parkinson's disease and improving Parkinson's symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
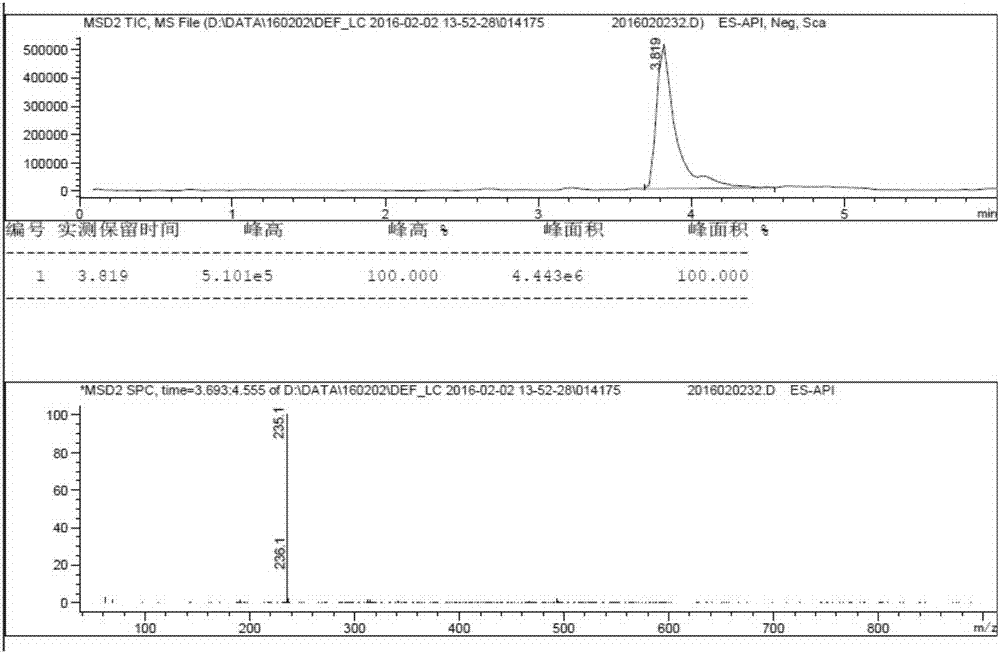
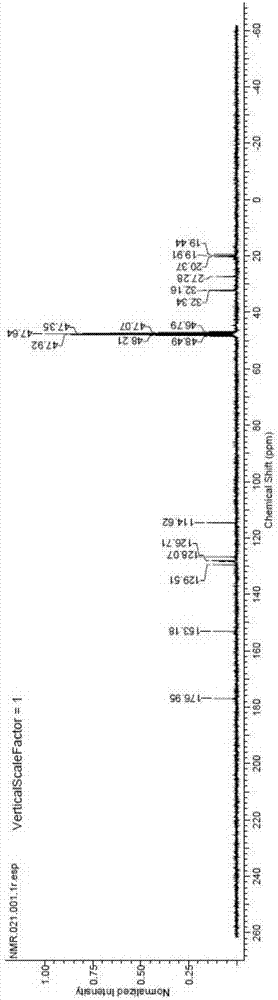
[0049] Synthesize (R,S)-4-(2-hydroxy-5-methylphenyl)-5-methylhexanoic acid according to the following procedure
[0050]
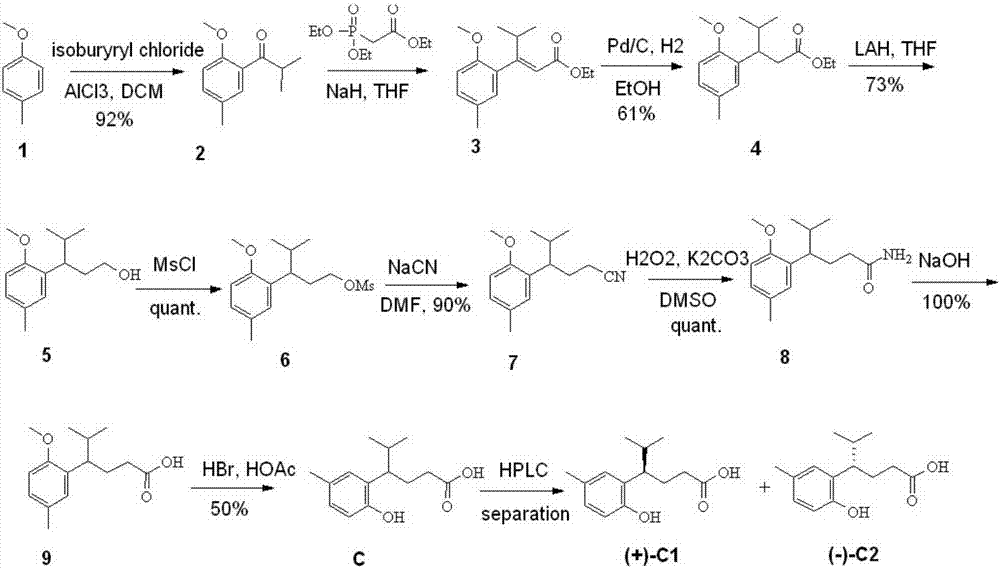
[0051] Add 1220g (10mol, 1eq) of p-methylanisole (2) into 12L of dichloromethane (room temperature), cool down to -5 to 0 degrees and add 1600g (12mol, 1.2eq) of aluminum trichloride in batches, After the addition, 1200g of succinic anhydride (12mol, 1.2eq) was added dropwise to the reaction system, and the temperature was controlled at about 10°C to react overnight. After the reaction is completed, add 10L of water dropwise to quench, separate the layers, extract the water phase with dichloromethane three times (5L*3), combine the dichloromethane phases, wash with 5% sodium bicarbonate solution and saturated brine until neutral (5L), dried over anhydrous sodium sulfate, dried with dichloromethane and spin-dried to obtain 1.6kg of crude product (3), which was used for the next reaction without purification.
[0052] Dissolve 1.6 kg of intermediate 3 in...
Embodiment 2
[0058] First prepare 4-(2-hydroxymethyl-5-ethylphenyl)-4-carbonyl butyric acid methyl ester according to the method similar to Example 1, then 1.4 kg of 4-(2-hydroxyl Methyl-5-ethylphenyl)-4-carbonylbutanoic acid methyl ester was dissolved in 15L tetrahydrofuran at room temperature, and the tetrahydrofuran solution (2.0M, 4.5L) of ethylmagnesium chloride was slowly added under temperature control below 0 degrees, and the dropwise after reaction. After the reaction is complete, add 3L of sulfuric acid to quench the reaction, stir for 10 hours, concentrate to remove most of the tetrahydrofuran, add 10L of dichloromethane, separate layers, extract the aqueous phase with dichloromethane three times, (5L*3), combine dichloromethane The methane phase was washed with 5% sodium bicarbonate solution and saturated brine to neutrality (5 L), dried over anhydrous sodium sulfate, and the dichloromethane phase was dried by spinning to obtain 0.8 kg of crude product, which was used for the n...
Embodiment 3
[0062] First prepare 4-(2-hydroxymethyl-5-propylphenyl)-4-carbonyl butyric acid methyl ester according to the method similar to Example 1, then 1.4 kg of 4-(2-hydroxyl Methyl-5-propylphenyl)-4-carbonylbutanoic acid methyl ester was dissolved in 15L tetrahydrofuran at room temperature, and the tetrahydrofuran solution (2.0M, 4.5L) of ethylmagnesium chloride was slowly added under temperature control below 0 degrees, and the dropwise after reaction. After the reaction is complete, add 3L of concentrated sulfuric acid to quench the reaction, stir for 10 hours, concentrate to remove most of THF, add 10L of dichloromethane, separate layers, extract the aqueous phase three times with dichloromethane (5L*3), and combine the two Chloromethane phase, washed with 5% sodium bicarbonate solution and saturated brine until neutral (5L), dried over anhydrous sodium sulfate, dichloromethane phase was dried and spin-dried to obtain 0.75kg of crude product, which was used for the next reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com