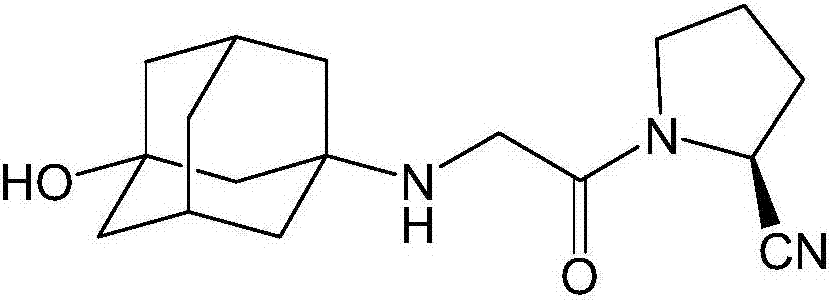
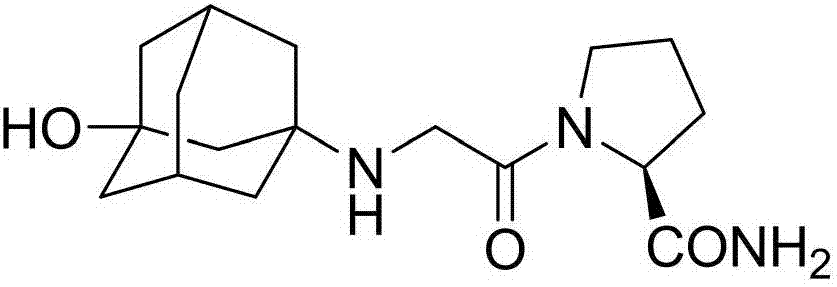
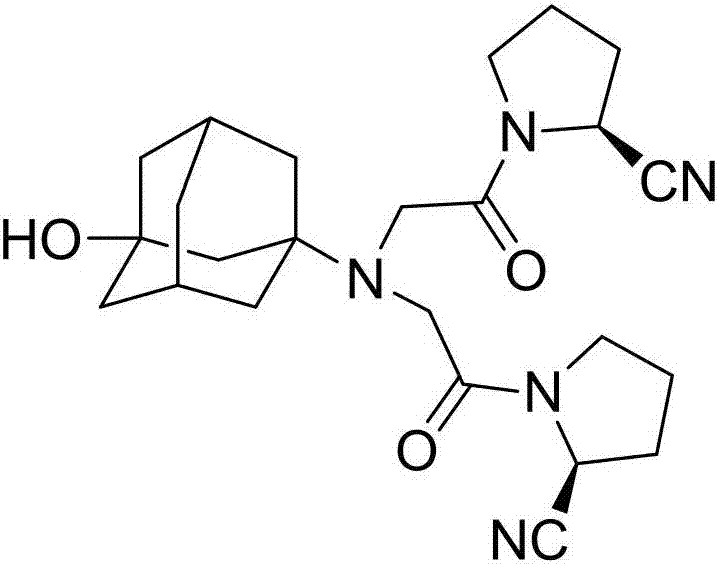
Preparation method of vildagliptin dimer impurities
A dimer and impurity technology, applied in the field of vildagliptin dimer impurity preparation, to achieve the effect of mild reaction conditions, high purity, and high impurity yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of preparation method of vildagliptin dimer impurity that the present invention proposes, the steps are as follows:
[0031] Potassium carbonate, catalyst and vildagliptin were dissolved in tetrahydrofuran, the molar ratio of vildagliptin and potassium carbonate was 1:2, vildagliptin intermediate (S)-N-chloroacetyl-2-cyano The base pyrrolidine is dissolved in tetrahydrofuran, and is added dropwise in the vildagliptin solution, and the mol ratio of vildagliptin and vildagliptin intermediate (S)-N-chloroacetyl-2-cyanopyrrolidine is 1:2, the dropwise addition was completed, refluxed for 20 hours, filtered, and the filtrate was concentrated to dryness to obtain the crude product of the target product, which was purified.
Embodiment 2
[0033] A kind of preparation method of vildagliptin dimer impurity that the present invention proposes, the steps are as follows:
[0034] Potassium carbonate, catalyst and vildagliptin were dissolved in tetrahydrofuran, the molar ratio of vildagliptin and potassium carbonate was 1:4, vildagliptin intermediate (S)-N-chloroacetyl-2-cyano The base pyrrolidine is dissolved in tetrahydrofuran, and is added dropwise in the vildagliptin solution, and the mol ratio of vildagliptin and vildagliptin intermediate (S)-N-chloroacetyl-2-cyanopyrrolidine is 1:3.5, the dropwise addition was completed, refluxed for 22 hours, filtered, the filtrate was concentrated to dryness, and the crude product of the target product was obtained; the crude product of the target product was dissolved in saturated saline, extracted with ethyl acetate, the ethyl acetate layer was separated, and dichloro Extract with methane, separate the water layer, combine the dichloromethane layers, wash with saturated bri...
Embodiment 3
[0036] A kind of preparation method of vildagliptin dimer impurity that the present invention proposes, the steps are as follows:
[0037] Potassium carbonate, catalyst and vildagliptin were dissolved in tetrahydrofuran, the molar ratio of vildagliptin and potassium carbonate was 1:5, vildagliptin intermediate (S)-N-chloroacetyl-2-cyano The base pyrrolidine is dissolved in tetrahydrofuran, and is added dropwise in the vildagliptin solution, and the mol ratio of vildagliptin and vildagliptin intermediate (S)-N-chloroacetyl-2-cyanopyrrolidine is 1:4, the dropwise addition was completed, refluxed for 26 hours, filtered, the filtrate was concentrated to dryness, and the crude product of the target product was obtained; the crude product of the target product was dissolved in saturated saline, extracted with ethyl acetate, the ethyl acetate layer was separated, and dichloride was added Extract with methane, separate the water layer, combine the dichloromethane layers, wash with sat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com