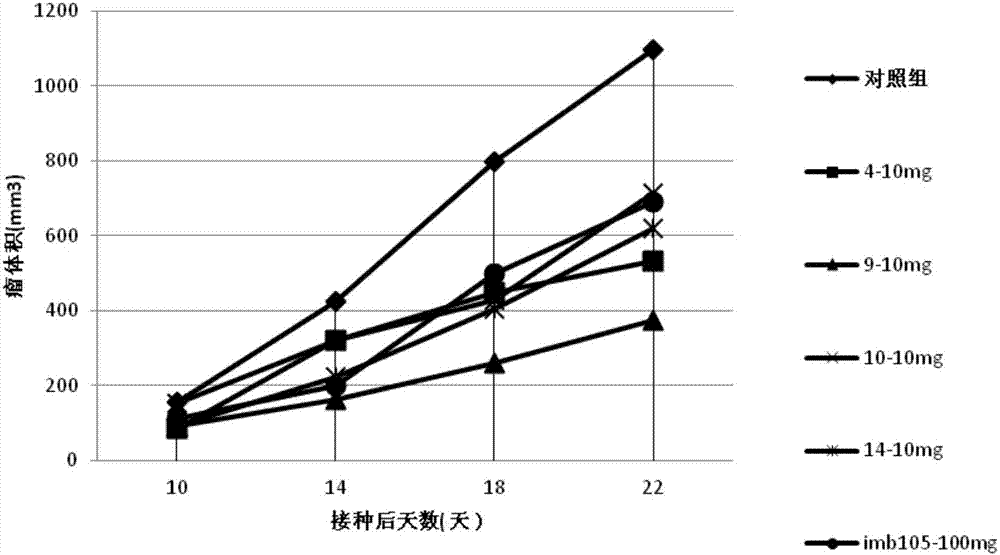
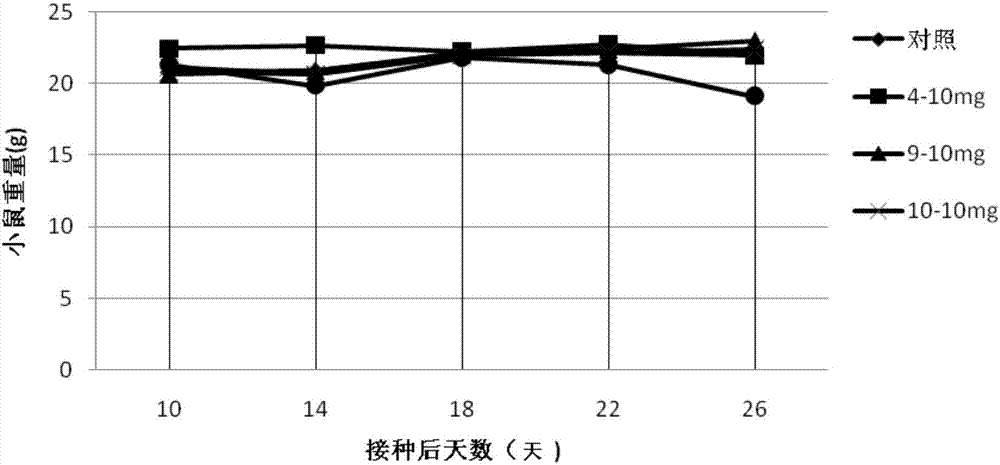
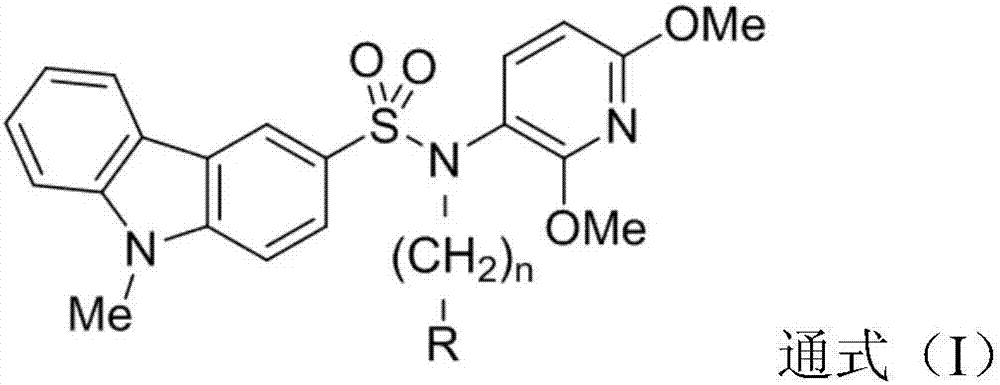
Carbazole sulfamide derivative or pharmaceutical slat thereof as well as preparation method and application thereof
A technology of carbazole sulfonamide and derivatives, which is applied in the field of medicine and can solve the problems of neurotoxic side effects, poor bioavailability, limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1: N-(2,6-dimethoxypyridin-3-yl)-N-(acetamido-2-yl)-9-methyl-3-carbazolesulfonamide (1)
[0105] N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide (IMB105) was prepared according to Mitsumori, Susumu; Tsuri, Tatsuo; Honma, Tsunetoshi et al., Journal of Synthesized by the method in Medicinal Chemistry (2003), 46 (12), 2436-2445. Dissolve N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide (IMB105) (0.30 g, 0.78 mmol) in 5 mL of anhydrous DMF, add iodine Acetamide (0.20 g, 1.1 mmol) and sodium hydride (40 mg, 60% in oil, 1.0 mmol) were reacted at 70° C. for 8 h, and the reaction was complete as detected by TLC. DMF was removed under reduced pressure, the residue was extracted with dichloromethane, washed successively with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was separated and purified by column chromatography (CDM / MeOH / concentrated ammonia water=40 / 1 / 0.1) to obtain a solid 0.35 g (98% yi...
Embodiment 2
[0106] Example 2: N-(N,N-dimethylethyl)-N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide (4)
[0107] (1) Preparation of N-(2-bromoethyl)-N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide(2)
[0108] Dissolve IMB105 (1.0g, 2.5mmol) in 20mL of anhydrous tetrahydrofuran, add bromoethanol (0.22mL, 3.0mmol), triphenylphosphine (1.3g, 5.0mmol), and add DEAD (azo Diethyl diformate 40% intoluene, 2.0 mL, 4.25 mmol), stirred for about 10 min, and stirred at room temperature until the reaction was detected by TLC (about 5 h). Ethyl acetate (50 mL) was added to the reaction solution, washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and separated and purified by column chromatography (PE / AcOEt=4 / 1-2 / 1) to obtain 1.2 g of solid (product rate 95%). 1 HNMR (CDCl 3 ,400MHz)δppm 3.39(3H,s),3.51(2H,t,J=7.2Hz),3.90(3H,s),3.96(5H,br s),6.36(1H,d,J=8.4Hz), 7.37(1H,t,J=8.4Hz),7.45(2H,d,J=8.8Hz),7.51(1H,d,J=8.4Hz),7.61(1H,dd,J=8.8,1....
Embodiment 3
[0111] Example 3: N-(2,6-dimethoxypyridin-3-yl)-N-[2-(pyrrol-1-yl)-ethyl]-9-methyl-3-carbazolesulfonamide (5)
[0112] The synthesis method is the same as that of the above-mentioned compound (4). N-(2-bromoethyl)-N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide (2) (0.5g, 1.0mmol) and Tetrahydropyrrole (0.42mL, 5.0mmoL) was reacted, separated and purified by column chromatography (DCM / MeOH / concentrated ammonia water=20 / 1 / 0.1) to obtain an oily substance. 1 HNMR (CDCl 3,400MHz)δppm 1.60(4H,brs),2.48(4H,brs),2.58(2H,brs),3.40(3H,s),3.82(2H,brs),3.88(3H,s),3.91 (3H,s),6.32(1H,d,J=8.4Hz),7.35(1H,t,J=8.0Hz),7.44(2H,d,J=8.8Hz),7.50(1H,d,J=8.0Hz),7.50(1H,d,J=8.0Hz) 8.4Hz), 7.54(1H,d,J=8.0Hz), 7.59(1H,t,J=8.4Hz), 7.83(1H,dd,J=8.0,1.6Hz), 8.13(1H,d,J= 8.0Hz), 8.47(1H,d,J=1.6Hz); HRMS(ESI+) 495.2070, Calcd for C 26 h 31 N 4 o 4 S495.2061[M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com