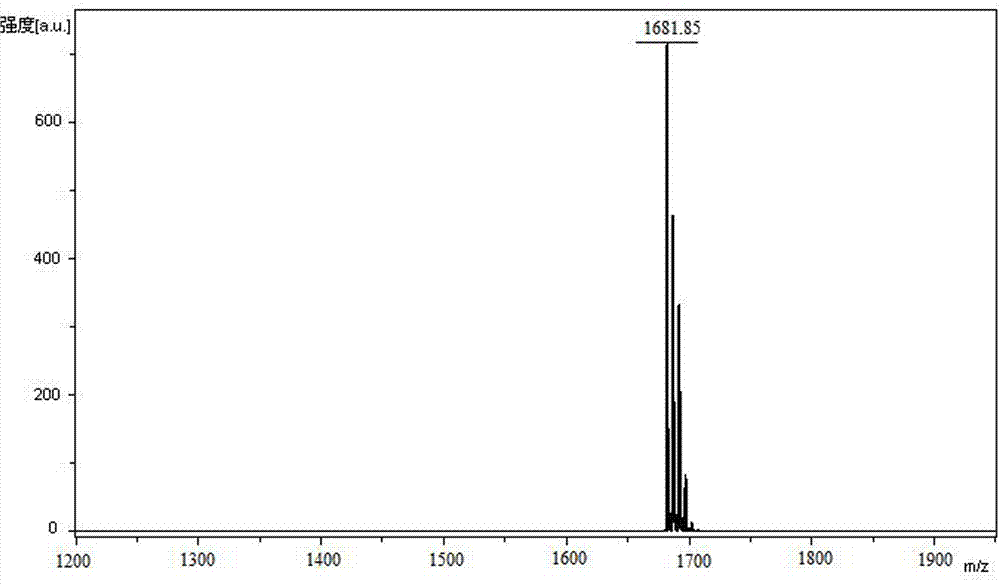
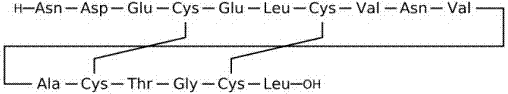
Method for solid phase synthesis of plecanatide by means of secondary cyclization
A technology of solid-phase synthesis and plecanatide, which is applied in peptide preparation methods, chemical instruments and methods, peptides, etc., can solve problems such as low efficiency, cumbersome extraction process, and difficulty in obtaining high-purity products, and achieve directional efficiency High, high product yield, conducive to the effect of product purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Synthesis of Fmoc-Leu-king resin with a degree of substitution of 0.10mmol / g
[0056] Weigh 20g of Wang resin with a degree of substitution of 0.45mmol / g, add to the solid phase reaction column, add to the solid phase reaction column, wash once with DMF, swell the resin with DMF for 30 minutes, and take 15.81g Fmoc-Leu -OH (45mmol), 6.01g HOBt (45mmol) dissolved in DMF, add 7.0ml DIC (45mmol) under ice water bath to activate, add to the above reaction column with resin, add 2.75g DMAP (22.5mmol) after 5 minutes After reacting for 2 hours, it was washed with DMF 3 times, DCM washed 3 times, capped with 100ml acetic anhydride / pyridine overnight, and methanol was shrunk and dried to obtain Fmoc-Leu-king resin with a detection degree of substitution of 0.10mmol / g.
Embodiment 2
[0057] Example 2: Synthesis of Fmoc-Leu-king resin with a degree of substitution of 0.50mmol / g
[0058] Weigh 10g of Wang resin with a degree of substitution of 1.50mmol / g, add to the solid phase reaction column, add to the solid phase reaction column, wash once with DMF, swell the resin with DMF for 30 minutes, and take 26.48g Fmoc-Leu -OH (75mmol), 10.13g HOBt (75mmol) dissolved in DMF, add 11.6ml DIC (75mmol) under ice water bath to activate, add to the above reaction column with resin, add 4.5g DMAP (37.5mmol) after 5 minutes After reacting for 2 hours, it was washed 3 times with DMF and 3 times with DCM, capped overnight with 100ml of acetic anhydride / pyridine, and methanol was shrinked and dried to obtain 22.54g of Fmoc-Leu-king resin. The detection degree of substitution was 0.50mmol / g.
Embodiment 3
[0059] Example 3: Preparation of fully protected pukanatide linear peptide king resin
[0060] Weigh 10.00g (1mmol) of Fmoc-Leu-King resin with a degree of substitution of 0.10mmol / g, add it to the solid phase reaction column, wash with DMF once, swell Fmoc-Leu-King resin with DMF for 30 minutes, then use DMF : The mixed solution of 4:1 volume ratio of pyridine removes Fmoc protection, then washes with DMF 6 times, weighs 2.07g Fmoc-Cys(Acm)-OH (5mmol), 0.68g HOBt (5mmol) and adds volume ratio of 1 :1 mixed solution of DCM and DMF, add 0.8ml DIC (5mmol) under ice-water bath for activation, add it to the above reaction column containing resin, react for 2 hours at room temperature, then detect the end of the reaction by ninhydrin method, if If the resin is colorless and transparent, it means that the reaction is complete; if the resin is colored, it means that the reaction is incomplete and requires another 1 hour to react. This criterion is suitable for subsequent amino acid coup...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com