Targeted PD-L1 mosaic antigen receptor modified T lymphocyte as well as preparation method and application thereof
A chimeric antigen receptor, lymphocyte technology, applied in genetically modified cells, cells modified by introducing foreign genetic material, botanical equipment and methods, etc., to achieve the effect of eliminating immunosuppressive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
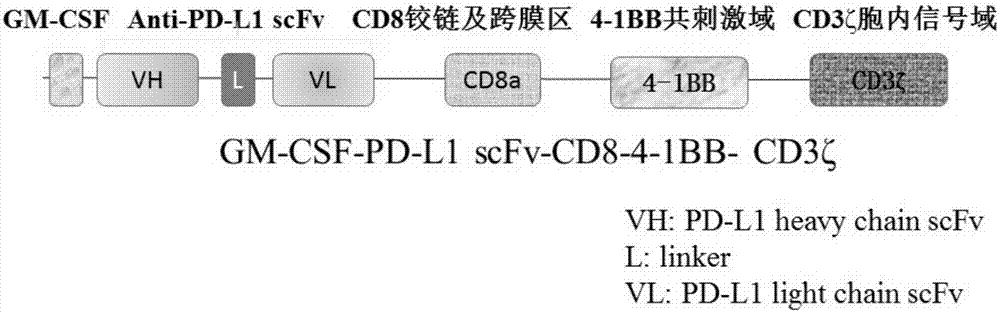
[0036] Example 1 Determination of the PD-L1-CAR gene sequence
[0037] 1.1 The GM-CSF signal peptide gene, human CD8α hinge region gene, human CD8α transmembrane region, 4 - 1BB co-stimulatory signal domain and CD3ζ intracellular signal domain gene sequence. The anti-PD-L1 single-chain antibody (anti-PD-L1 scFv) gene sequence comes from a patent (patent publication number: CN105793288A). See the sequence listing for the sequence information of each gene.
[0038] 1.2 The above gene sequences were sequentially linked according to the human GM-CSF signal peptide gene, anti-PD-L1 scFv, human CD8α hinge region gene, human CD8α transmembrane region, 4-1BB co-stimulatory signal domain and CD3ζ intracellular signal domain gene sequence, Form the final complete PD-L1-CAR gene sequence (the nucleotide sequence of PD-L1-CAR) information.
Embodiment 2
[0039] Example 2 Construction of PD-L1-CAR expression plasmid
[0040] 2.1 Whole gene synthesis:
[0041] The complete PD-L1-CAR sequence is synthesized from the whole gene, and enzyme cleavage sites are added at both ends.
[0042] 2.2 Use primers PD-L1-F and PD-L1-R to amplify the complete PD-L1-CAR gene sequence by PCR. The PCR system is:
[0043]
[0044] PCR reaction conditions: 94°C for 4 minutes; 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 90 seconds, 34 cycles; 72°C for 5 minutes;
[0045] Run the PCR product on an agarose gel for fragment size identification, and perform gel recovery to obtain the complete PD-L1-CAR gene sequence.
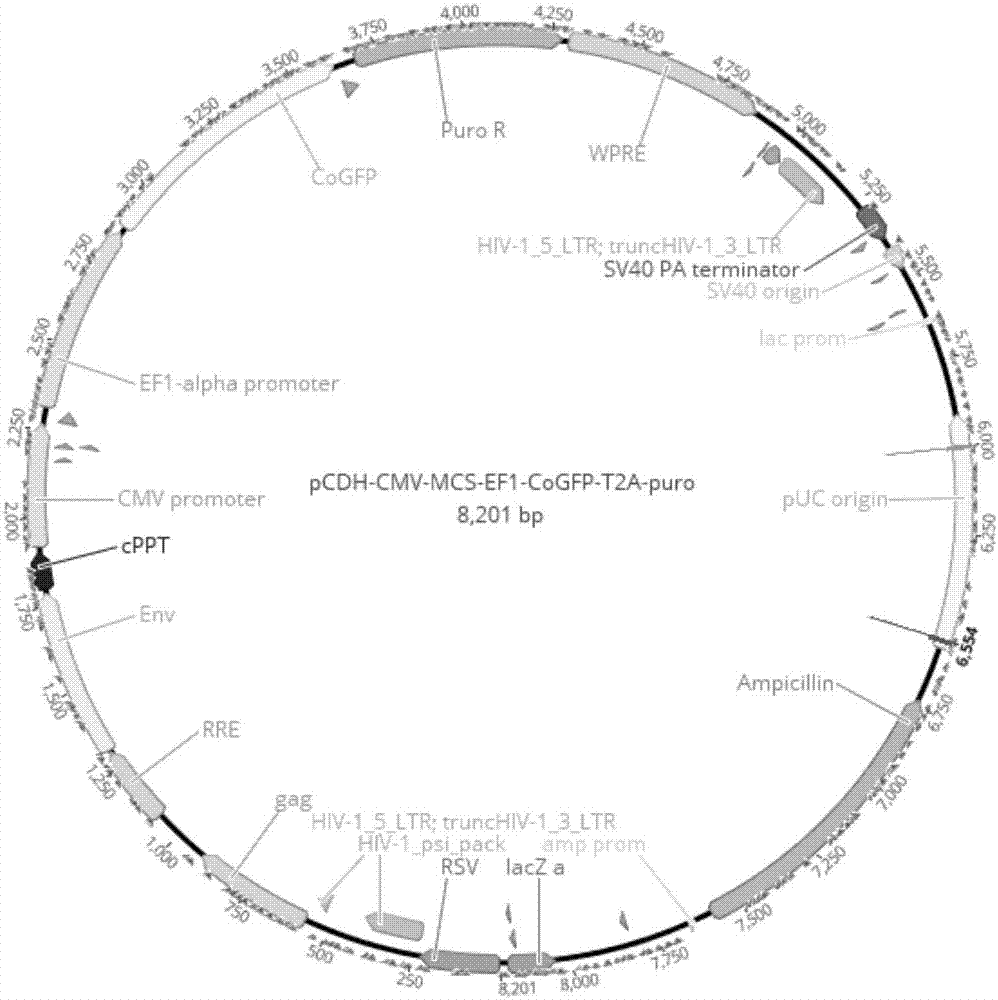
[0046] 2.3 Cloning the above sequence into pCDH-CMV-MCS-EF1-copGFP-T2A-puro (pCDH for short) lentiviral expression vector to obtain the expression plasmid of anti-PD-L1-CAR.
[0047] Respectively digest PD-L1-CAR and lentiviral expression vector pCDH with XbaI / EcoRI, the enzyme digestion system is as follows, 37 ° C water bath...
Embodiment 3
[0054] Packaging, concentration and titer determination of embodiment 3 lentivirus
[0055] 3.1 Packaging of lentivirus:
[0056] 3.1.1 Cell treatment: 24 hours before transfection, collect 293T cells at passage 3-10 in logarithmic growth phase, inoculate 5×10^6 293T cells in a 10cm culture plate, and store the cells in DMEM containing 10ml 10% FBS Grow in culture medium at 37°C with 5% CO 2 Cultivate in the incubator for 24 hours, and transfection can be carried out when the density reaches 60-80%.
[0057] 3.1.2 Co-transfect the lentiviral vector plasmid (pCDH-PD-L1-CAR or its empty vector pCDH) and its packaging plasmid pLP1, pLP2, pLPVSV-G into the cells at a ratio of 5:2:2:2; The dyeing system is as follows:
[0058]
[0059] After 6-8 hours, replace the medium in the 10 cm culture dish with complete medium.
[0060] 3.1.3 24 hours after transfection, the expression of GFP fluorescence in 293T cells after transfection was observed under a fluorescent microscope. C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com