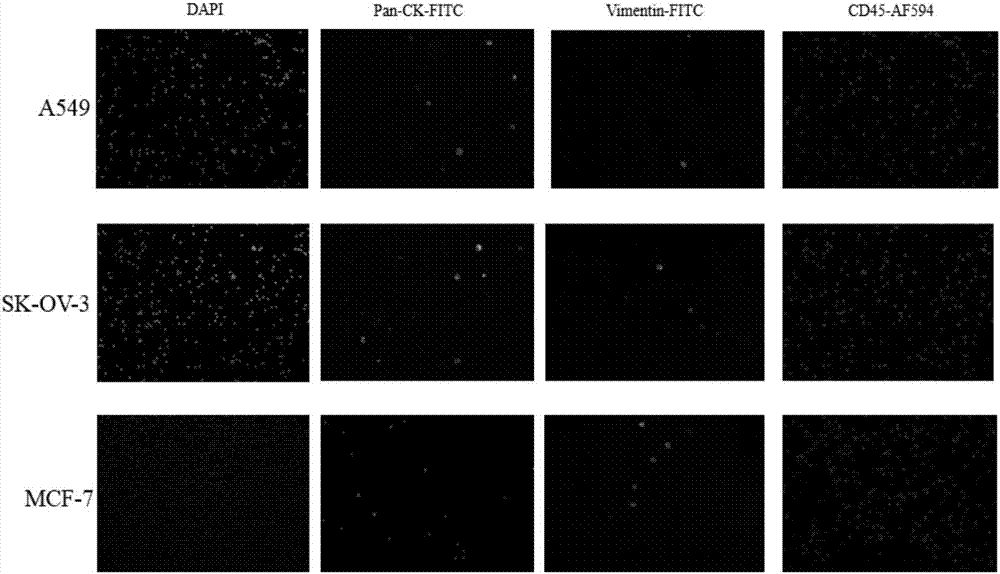
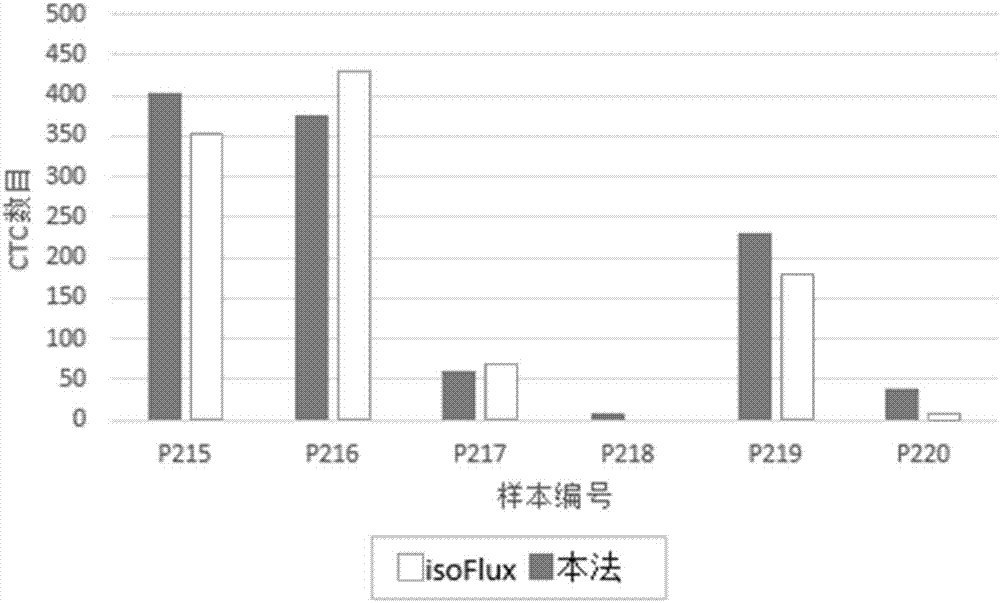
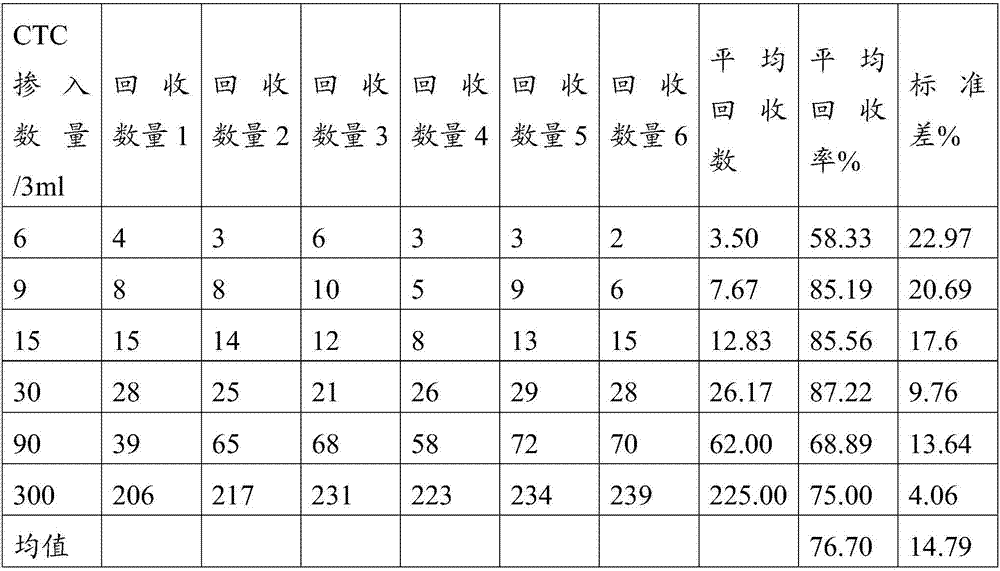
Immunofluorescent staining and interpretation method for circulating tumor cell
An immunofluorescence staining and tumor cell technology, applied in the field of immunofluorescence staining and interpretation of circulating tumor cells, can solve the problems of complex operation, difficult popularization, and increased difficulty for clinical experimenters, and achieve accurate interpretation results, extended recognition range, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0176] Embodiment 4 of the present invention is: a method for immunofluorescent staining of circulating tumor cells, comprising the following steps:
[0177] S10 sample processing: after the blood sample diluted with phosphate buffer solution is centrifuged, red blood cells and white blood cells in the blood sample are removed;
[0178] S20 pre-staining treatment: Rinse the blood sample treated in step S10 with phosphate buffer solution, add fixative solution to fix the cells in the blood sample, add permeabilization solution for permeabilization, and then add blocking solution to fully seal;
[0179] S30 Immunostaining: Add anti-pan-CK-FITC staining solution, anti CD45-AF594 staining solution and anti-Vimentin-FITC staining solution dropwise to the blood sample treated in step S20 in the dark, incubate in the dark and discard Add nucleic acid fluorescent dye to the above staining solution to continue incubation.
[0180] In the step S10, the blood sample diluted one-fold wit...
Embodiment 5
[0186] Embodiment 5 of the present invention is: a method for immunofluorescent staining of circulating tumor cells, comprising the following steps:
[0187] S10 sample processing: after the blood sample diluted with phosphate buffer solution is centrifuged, red blood cells and white blood cells in the blood sample are removed;
[0188] S20 pre-staining treatment: Rinse the blood sample treated in step S10 with phosphate buffer solution, add fixative solution to fix the cells in the blood sample, add permeabilization solution for permeabilization, and then add blocking solution to fully seal;
[0189] S30 Immunostaining: Add anti-pan-CK-FITC staining solution, anti CD45-AF594 staining solution and anti-Vimentin-FITC staining solution dropwise to the blood sample treated in step S20 in the dark, incubate in the dark and discard Add nucleic acid fluorescent dye to the above staining solution to continue incubation.
[0190] In the step S10, the blood sample diluted 3 times with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com