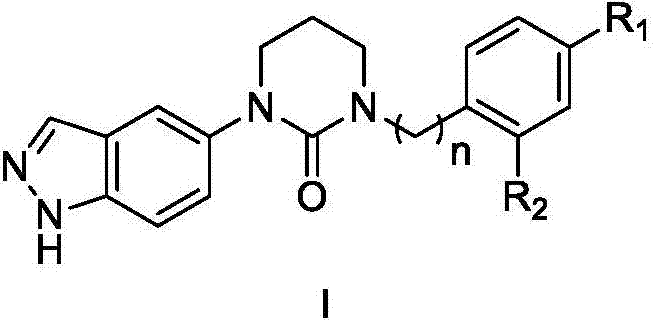
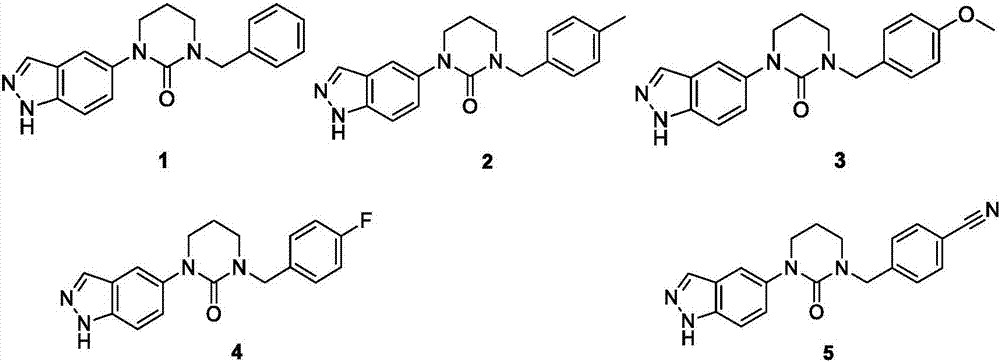
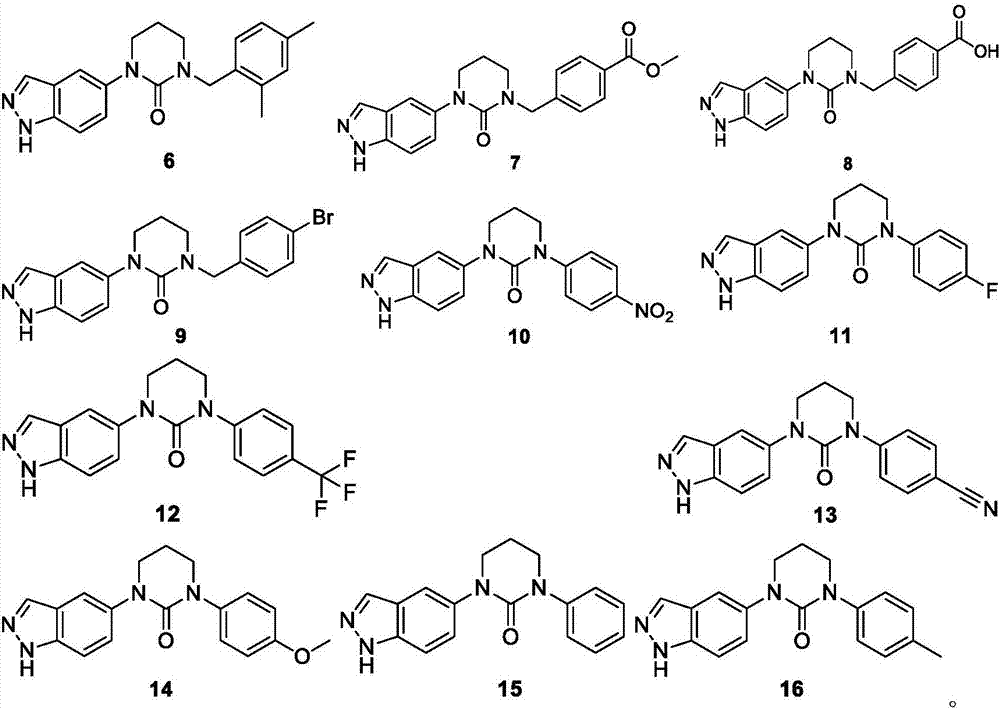
3-(1H-indazole)-tetrahydropyrimidine-2-one compounds, preparation method, and application thereof
A compound and composition technology, applied in the field of 3-tetrahydropyrimidin-2-one compounds, can solve the problems of no prevention and treatment of cardiovascular and cerebrovascular diseases, diabetes, etc., achieve good application and development prospects, and protect blood vessels Function, the effect of promoting glucose consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
[0044] The preparation of embodiment 1-16 intermediate:
[0045] Step A: 5-aminoindazole (474mg, 3.56mmol), 6ml of anhydrous pyridine in dichloromethane (6ml) was added to a 25ml round bottom flask, and methyl chlorobenzoate (0.45ml, 3.56ml) was slowly added dropwise at 80°C. mmol). The reaction was stirred at room temperature. After reacting for about 6 hours, TLC showed that the starting material basically disappeared, so the reaction was stopped, and the solvent was removed under reduced pressure. Silica gel column chromatography (dichloromethane: ethyl acetate = 50:1) gave 812 mg of the product with a yield of 90%.
[0046]
[0047] 1 H NMR (400MHz, DMSO, δppm) 12.98(s, 1H), 10.17(s, 1H), 8.01(s, 1H), 7.92(s, 1H), 7.50(d, J=8.8Hz, 1H), 7.42 (t,J=8.4Hz,3H),7.24(m,3H).
[0048] HR-MS(ESI)m / z(M+H) + calcd for C 14 h 12 N 3 o 2 :254.09240; Found: 254.09120.
[0049] Step B: add compound 17 (100mg, 0.4mmol) in 10ml round bottom flask, 2ml anhydrous THF (6ml), stir...
Embodiment 1
[0058] The synthesis of embodiment 1 compound 1
[0059] Step A: Add compound 19 (200mg, 0.67mmol), 4ml of anhydrous 1,4-dioxane to a 25ml round bottom flask, add NaH (44mg, 1.82mmol) at 0°C, benzyl bromide (124mg, 0.73ml ). Stirring at room temperature for about 3 hours, TLC showed that the starting material basically disappeared, the reaction was stopped, and the solvent was removed under reduced pressure. Dilute with dichloromethane, wash with saturated sodium bicarbonate solution, water and saturated sodium chloride solution successively, and dry over anhydrous sodium sulfate. Filtration, concentration, and silica gel column chromatography (dichloromethane:methanol=100:1) gave 223 mg of the product with a yield of 86%.
[0060]
[0061] 1 H NMR (400MHz,DMSO,δppm)8.05(s,1H),7.63(m,2H),7.35(m,3H),7.28(m,3H),5.81(m,1H),4.51(s,2H) ,3.85(m,1H),3.71(m,3H),3.28(m,2H),2.40(m,1H),1.97(m,4H),1.73(m,1H),1.57(m,2H).
[0062] HR-MS(ESI)m / z(M+H) + calcd for C 23 h 27 N 4 o ...
Embodiment 2
[0067] The synthesis of embodiment 2 compound 2
[0068] Step A: Add compound 19 (80mg, 0.27mmol), 4ml of anhydrous 1,4-dioxane to a 25ml round bottom flask, add NaH (10mg, 0.40mmol) at 0°C, p-methylbenzyl bromide (59mg , 0.32 mmol). Stirring at room temperature for about 3 hours, TLC showed that the starting material basically disappeared, the reaction was stopped, and the solvent was removed under reduced pressure. Dilute with dichloromethane, wash with saturated sodium bicarbonate solution, water and saturated sodium chloride solution successively, and dry over anhydrous sodium sulfate. After filtration, concentration, and silica gel column chromatography (dichloromethane:methanol=100:1), 78mg of the product was obtained with a yield of about 72%.
[0069]
[0070] 1H NMR (400MHz, DMSO, δppm) δ8.04(s, 1H), 7.64(d, J=9.0Hz, 1H), 7.60(s, 1H), 7.35(d, J=9.0Hz, 1H), 7.17 (m,4H),5.82(d,J=10.0Hz,1H),4.45(s,2H),4.07(q,J=5.3Hz,1H),3.87(d,J=11.4Hz,1H),3.73 (q,J=5.9,5.0Hz,1H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com