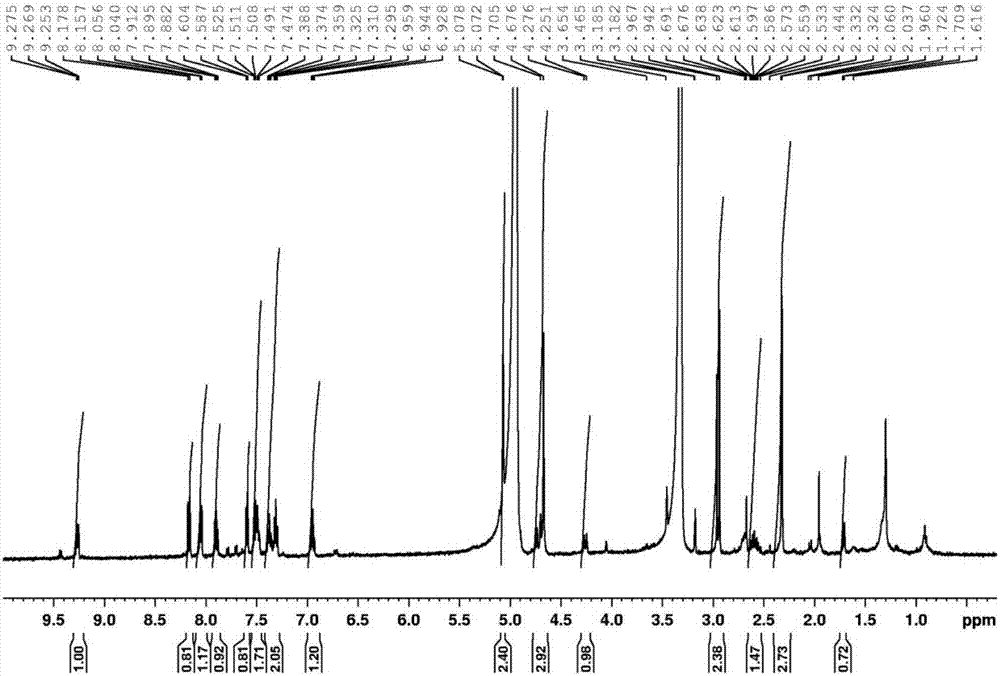
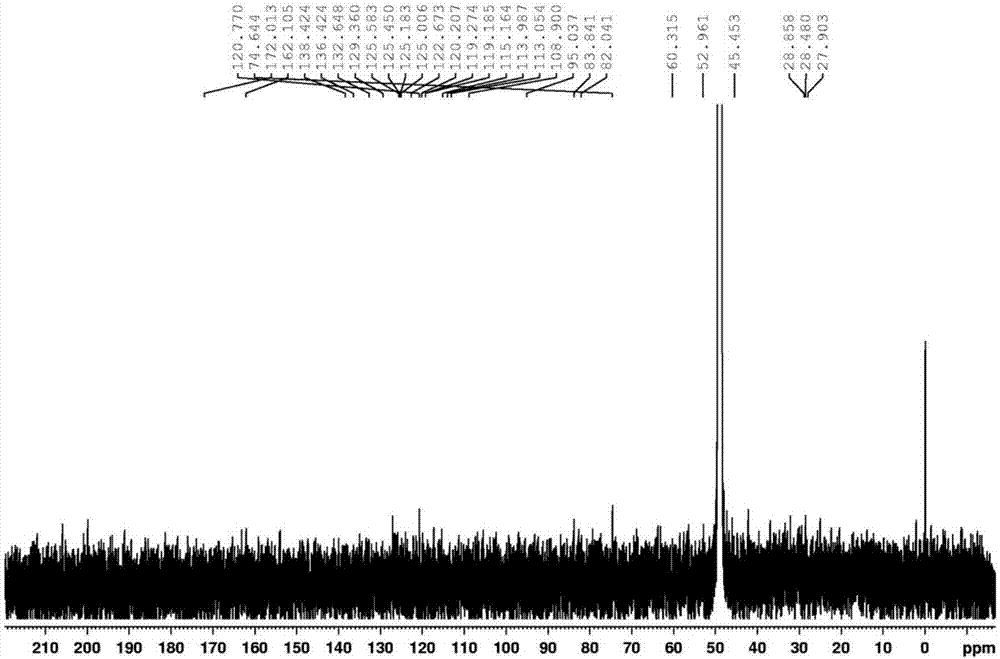
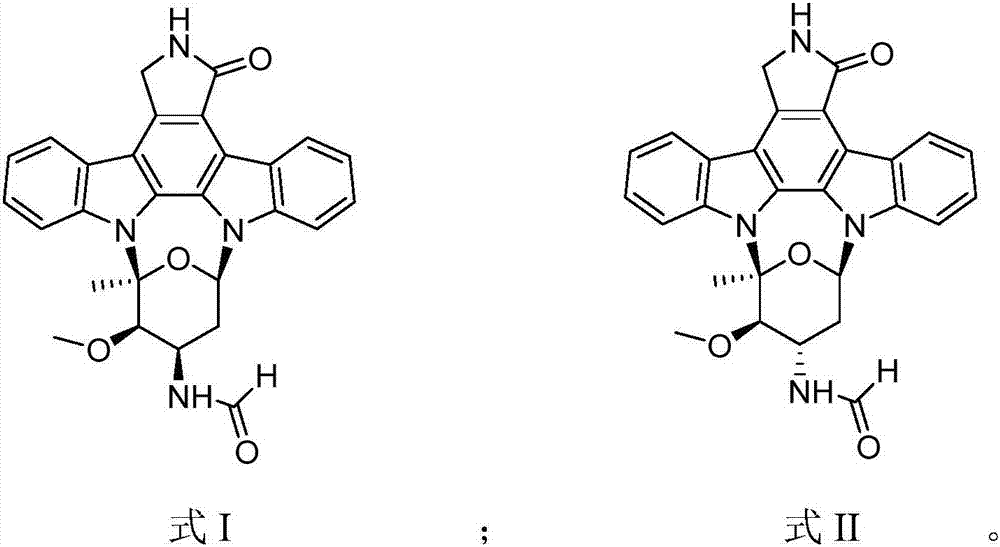
Staurosporine aldehyde substituted derivative as well as preparation method and application thereof
A technology of staurosporine aldehyde and derivatives, applied in the field of staurosporine aldehyde substituted derivatives and its preparation, can solve the problems of high toxicity, no specificity of Staurosporine, and influence on normal cell proliferation and division, and achieve experimental operation Simple, easy to expand production, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Fermentation of compounds
[0026] Marine actinomycetes use Streptomycessp.CICC 11027 sold by China Common Microbial Species Collection and Management Center;
[0027] 1) Inoculate marine actinomycetes in a 500mL Erlenmeyer flask, each flask containing 250mL Gao's No. 1 liquid medium, culture condition is 28 ℃, 180rpm shaker for 3 days to obtain seed liquid that can be used for fermentation culture ;
[0028] 2) Inoculate the seed liquid obtained in step 1) into rice culture medium (rice culture medium, made of the following components: 40g of rice mass; 60mL of seawater, placed in a 500ml Erlenmeyer flask and sterilized by autoclaving), inoculate The volume is 12 mL, and the solid fermentation product containing the compound with anti-tumor activity of the present invention is obtained by static culture at 28° C. for 120 days.
[0029] 2. Preparation of the compound
[0030] The solid fermentation product containing the compound with anti-tumor activity of the present inven...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com