Bacteriophage electrochemical luminescence signal amplification probe and preparation method and application thereof
A phage electrochemical and luminescent signal technology, applied in the field of biochemical analysis, can solve the problems of uncontrollable labeling, non-detection methods, and poor biological stability of nanomaterials, and achieve high signal amplification efficiency, stable system, and easy preservation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
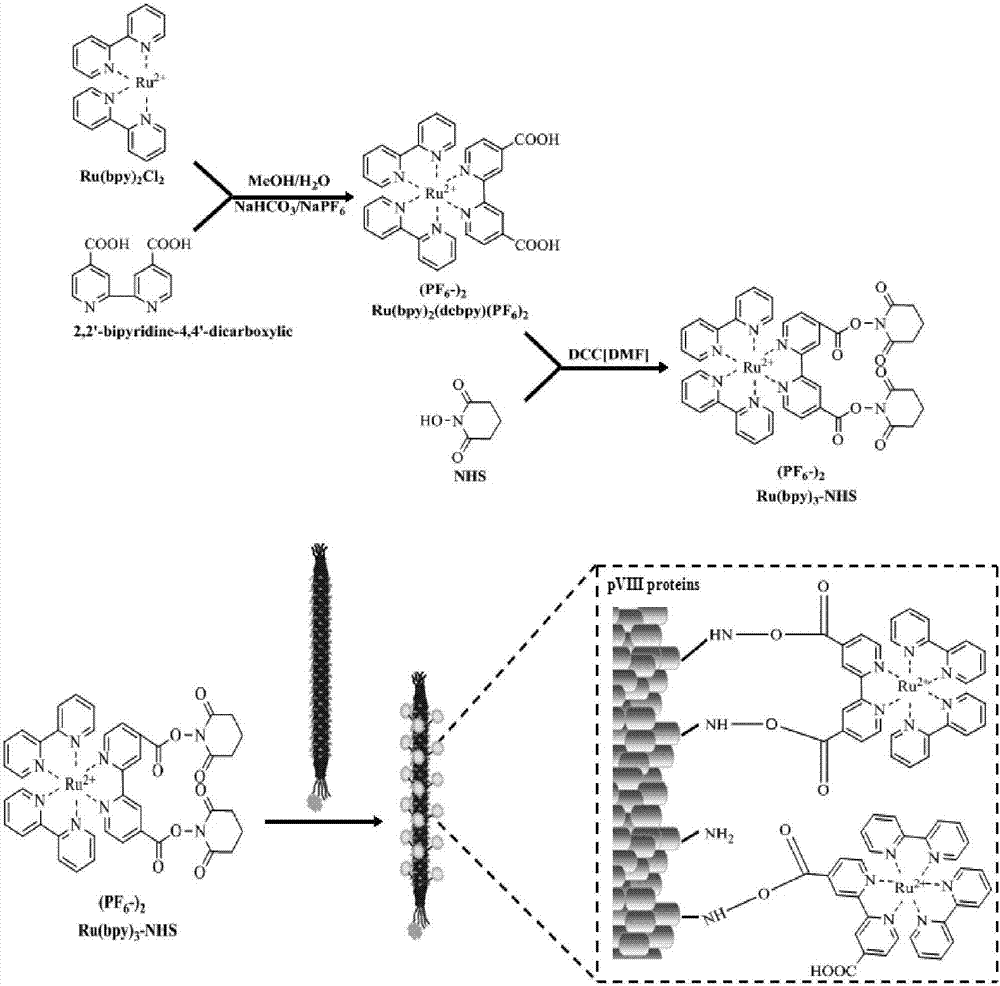
[0034] Embodiment one: Preparation of terpyridine ruthenium-N-hydroxysuccinimide ester (Ru(bpy) 3 2+ -NHS)
[0035] 1. Synthesis of terpyridine ruthenium hexafluorophosphate (Ru(bpy) 2 (dcbpy)(PF 6 ) 2 )
[0036] 0.2 g cis-dichlorobis(2,2′-bipyridyl)ruthenium(II), 0.15 g 2,2′-bipyridine-4,4′-dicarboxylic acid, 0.2 g sodium bicarbonate, 32 mL methanol and Add 8 mL of deionized water into a three-necked flask, install a condenser, heat to 80°C in a silicone oil bath under magnetic stirring, and heat to reflux for 10 hours. The reaction solution gradually changes from purple-brown to orange-red. After the reaction, the pH value of the reaction solution was adjusted to 4.4 with concentrated sulfuric acid, and the temperature was lowered in an ice bath for 2 hours in a dark environment to precipitate excess 2,2'-bipyridine-4,4'-dicarboxylic acid , and then vacuum-filtered, and the filtrate was collected to obtain the carboxylated terpyridine ruthenium filtrate. Add 12.5 mL o...
Embodiment 2
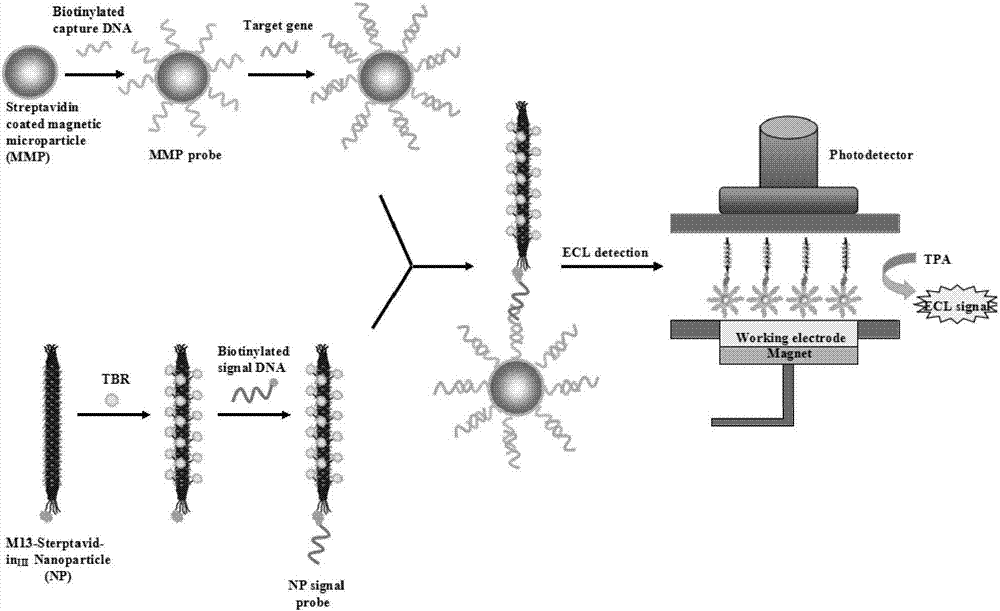
[0039] Example 2: Preparation of streptavidin-M13 phage
[0040] 1. Design specific primers, and clone the DNA fragment of streptavidin (SA) into the phagemid vector pC to obtain the plasmid pC-SA.
[0041] The DNA sequence of streptavidin (SA) is as follows (as shown in SEQ ID NO: 1 in the sequence listing):
[0042] atggacccctccaaggactcgaaggcccaggtctcggctgccgaggccggcatcaccggcacctggtacaaccagctcggctcgaccttcatcgtgaccgcgggcgccgacggcgccctgaccggaacctacgagtcggccgtcggcaacgccgagagccgctacgtcctgaccggtcgttacgacagcgccccggccaccgacggcagcggcaccgccctcggttggacggtggcctggaagaataactaccgcaacgcccactccgcgaccacgtggagcggccagtacgtcggcggcgccgaggcgaggatcaacacccagtggctgctgacctccggcaccaccgaggccaacgcctggaagtccacgctggtcggccacgacaccttcaccaaggtgaagccgtccgccgcctccatcgacgcggcgaagaaggccggcgtcaacaacggcaacccgctcgacgccgttcagcag。
[0043] Upstream primer: tataat GGCCCAGCCGGCC ATGGCCATGGACCCCTCCAAG (the underlined part is the SfiI restriction site);
[0044] Downstream primer: ataaa TGCGGCCGC CTGCTGAACGGCGTC (th...
Embodiment 3
[0058] Example 3: Preparation of streptavidin-M13 phage-ruthenium signal amplification probe
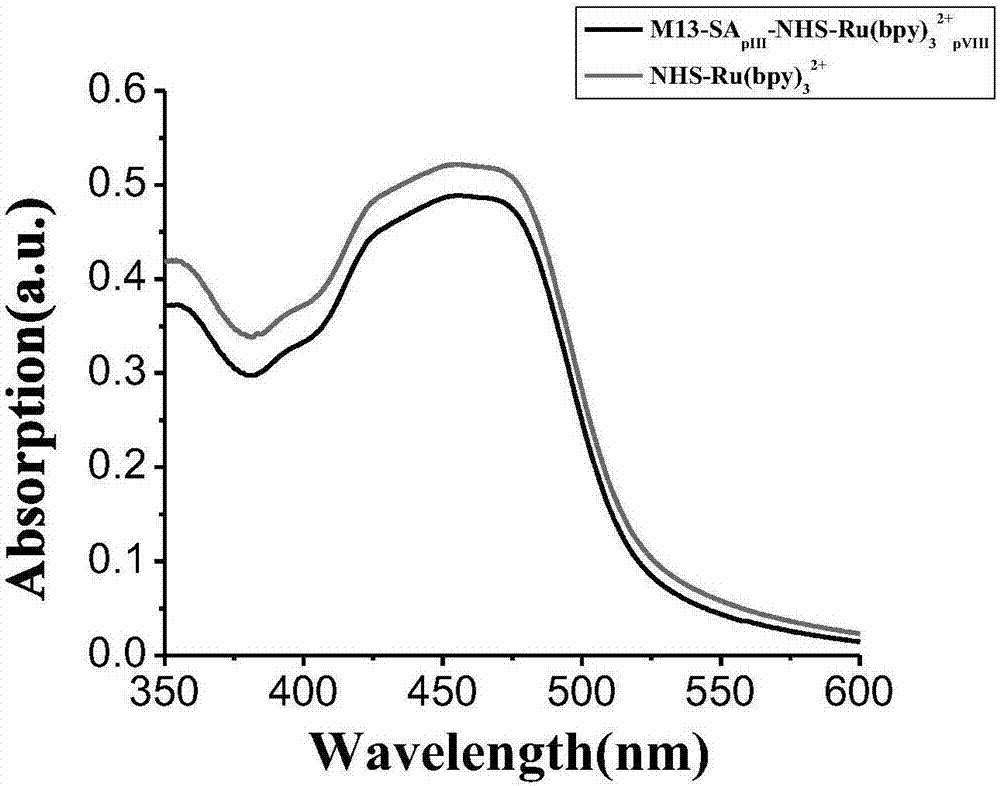
[0059] 100 μL of 10 12 Streptavidin-M13 phage at pfμ / mL was added to 20μL PEG / NaCl mixed solution (20%w / v PEG8000 / 2.5M NaCl) and mixed, placed on ice for 1 hour, and then centrifuged at room temperature at 11000rpm for 20 Minutes, the M13 phage pellets were obtained. Resuspend the M13 phage pellet in 100 μL of 0.2M NaHCO pH 8.3 3 In the buffer, add 5 μL of 10 mg / mL terpyridine ruthenium-N-hydroxysuccinimide ester (Ru(bpy) 3 2+ -NHS), and incubated overnight at 4°C with shaking in the dark. 10 μL of 1.5M hydroxylamine solution at pH 8.5 was added to the M13 phage solution and incubated at room temperature for 1 hour to terminate the labeling reaction. It was then filtered through PEG precipitation and a Zeba centrifugal desalting column to remove unreacted ruthenium-N-hydroxysuccinimide terpyridyl ester. After purification, streptavidin-M13 phage-ruthenium signal amplification p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com