Xanthene compound, colorant composition containing same, and resin composition containing same
A resin composition and compound technology, applied in the field of xanthene compounds, colorant compositions containing them and resin compositions containing them, can solve problems such as not simple, achieve small color change, excellent heat resistance and / or effect of chemical resistance, high degree of crosslinking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
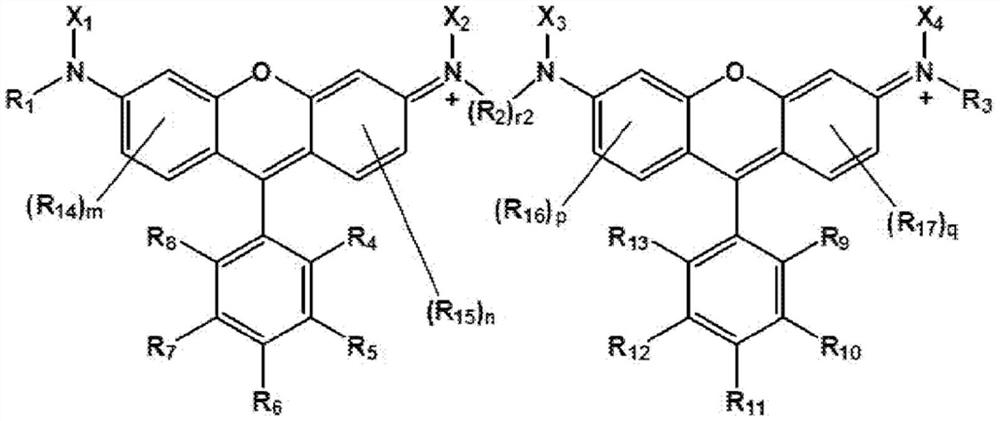
[0081] The compound of Chemical Formula 1 can be prepared with reference to the preparation examples described below.
[0082] Another embodiment of the present specification provides a colorant composition including the compound of Chemical Formula 1.
[0083] The colorant composition may further include at least one type of dye and pigment in addition to the compound of Chemical Formula 1. For example, the colorant composition may comprise only the compound of Formula 1, but may also comprise the compound of Formula 1 and one or more types of dyes, the compound of Formula 1 and one or more types of pigments, or The compound of Chemical Formula 1, one or more types of dyes, and one or more types of pigments are included.
[0084] Yet another embodiment of the present specification provides a resin composition including a colorant composition.
[0085] According to one embodiment of the present specification, the resin composition may include the compound represented by Chem...
preparation example 1
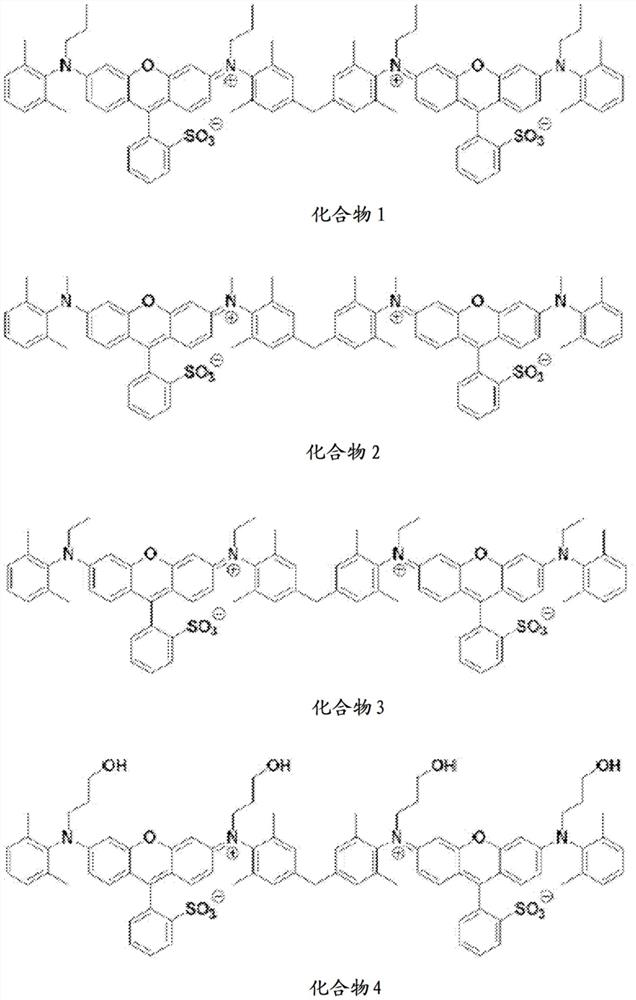
[0136] Preparation Example 1. Preparation of Compound 1
[0137]
[0138] 1) Preparation of compound A-3
[0139] Under shielding conditions, 3.000 g (7.403 mmol) of A-1 and 0.753 g (2.96 mmol) of A-2 were introduced into 30 g of N-methylpyrrolidone (NMP), and the resultant was reacted at 150° C. 4 hours. The solvent was removed under vacuum, and 1.174 g (1.184 mmol) of compound A-3 were obtained by subjecting the crude compound to column chromatography.
[0140] 2) Preparation of compound A-5
[0141]5.000 g (5.041 mmol) of Compound A-3 and 2.443 g (20.162 mmol) of Compound A-4 were introduced into 50 g of N-methylpyrrolidone (NMP), and the resultant was reacted at 150° C. for 5 hours. The resultant was cooled to room temperature, 35% HCl was added thereto, and the resultant was stirred. The solvent was removed under vacuum, and 1.756 g (1.512 mmol) of compound A-5 was obtained by column chromatography.
[0142] 3) Preparation of Compound 1
[0143] K 2 CO 3 It was...
preparation example 2 to 10
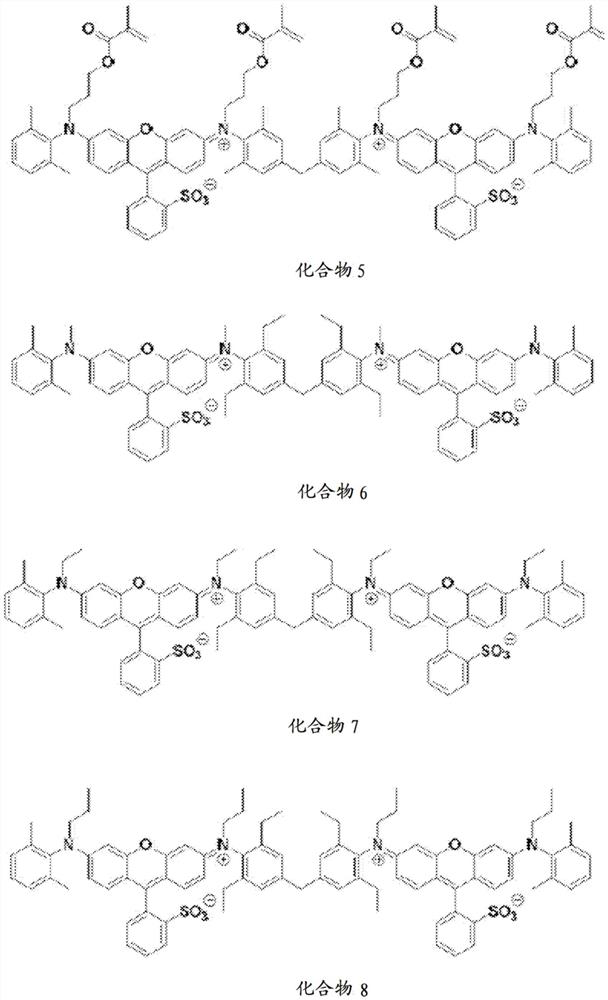
[0145] Preparation Examples 2 to 10. Preparation of Compounds 2 to 10
[0146]
[0147]
[0148]
[0149] Compounds 2 to 10 were prepared in the same manner as in Preparation Example 1 except that different substituents were used.
[0150] The compounds of Examples and Comparative Examples were prepared at the ratios listed in Table 1 below. Compounds are added in grams (g).
[0151] [Table 1]
[0152]
[0153] Substrate preparation
[0154] The compound prepared by the above synthesis was spin-coated on glass (5×5 cm) and prebaked at 100° C. for 100 seconds to form a film. The distance between the film-formed substrate and the photomask was 250 μm, and a stepper was used at 40 mJ / cm 2 The exposure dose irradiates the entire surface of the substrate. Thereafter, the exposed substrate was developed in a developing solution (KOH, 0.05%) for 60 seconds, and post-baked at 230° C. for 20 minutes to prepare a substrate.
[0155] Heat Resistance Evaluation
[0156]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com