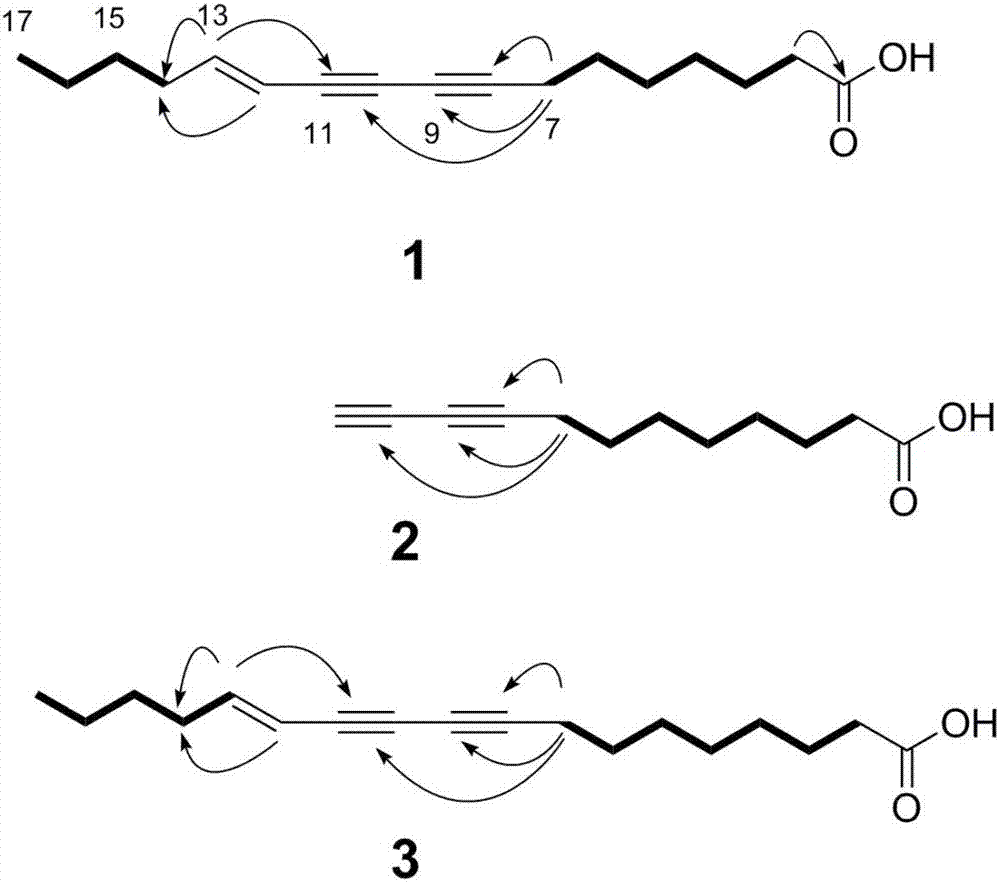
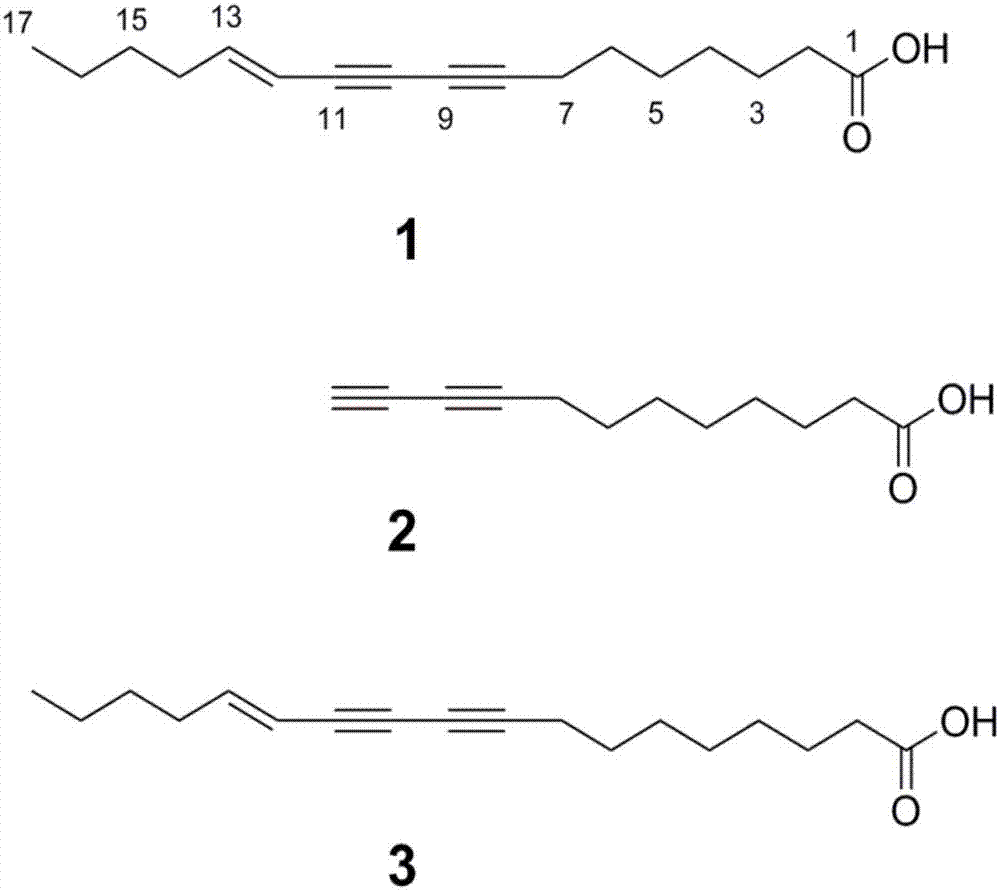
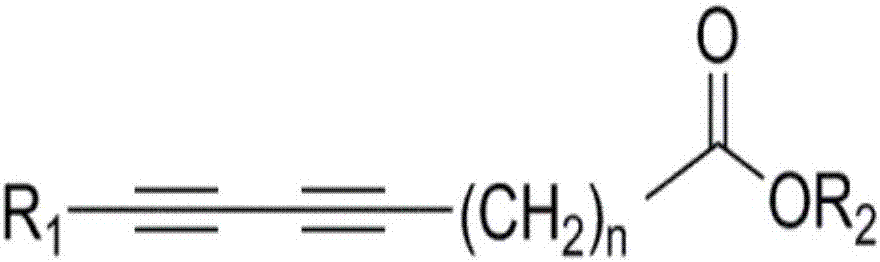Poly-acetylenic acid derivative
A technology of polyalkynoic acid and derivatives, which is applied in the field of medicine and can solve the problem of no active ingredients of thymegrass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 6Kg of the whole herb of Thyme chinensis, crush it, add 30L 95% ethanol to heat and reflux for extraction for 5 hours, repeat 4 times, filter and combine the extracts, recover the ethanol under reduced pressure, and suspend the extract in water.
[0038] Extract the suspension with petroleum ether (60-90°C) several times until the petroleum ether layer is nearly colorless, discard the petroleum ether layer; then extract the water layer with ethyl acetate saturated with water until the ethyl acetate layer is nearly colorless; The ethyl acetate layer was recovered under reduced pressure to obtain the ethyl acetate fraction. Through silica gel column chromatography, petroleum ether-ethyl acetate system was used successively, and the volume ratio was (100:1), (50:1), (20:1), (10:1), (5:1), ( 2:1), (1:1), (0:1) elution, collect (5:1) to (1:1) eluate, recover the solvent under reduced pressure, and obtain the extract. By ODS reverse phase column chromatography, gradient...
Embodiment 2
[0050] Detect the Thymus ethanol extract (EtOH extract) gained in Example 1, and the extracted petroleum ether fraction (PE fraction), the impact of ethyl acetate fraction (EtOAc fraction) on periodontitis pathogenic bacteria Porphyromonasgingivalis, the results show that the ethanol extract And ethyl acetate part has a certain inhibitory activity of periodontitis pathogenic bacteria Porphyromonas gingivalis.
[0051] According to the quality of the drug, 200mg / mL mother solution was formulated with DMSO, and after shaking, it was found that it could be dissolved without precipitation. The dilution test was started from the drug toxicity upper limit of 1000 μg / mL. At the beginning, the concentrations of the three samples were set at 1000, 500, 200, 100, and 50 μg / mL, respectively, and the initial concentration of Porphyromonas gingivalis was 10^7 / mL. They were co-cultured for 48 hours, and the growth of bacteria in each group was observed with the naked eye. Repeat 2 times. ...
Embodiment 3
[0055] Detect the impact of the three polyalkynoic acid components obtained in Example 1 on periodontal disease pathogenic bacteria (Porphyromonas gingivalis, Fusobacterium nucleatum), and the results show that polyalkynoic acid derivatives can inhibit periodontal disease pathogenic bacteria (Porphyromonas gingivalis, Fusobacterium nucleatum) ) growth. At the same time, it has no cytotoxicity to hGFs. It shows that this kind of compound has antibacterial effect and good safety.
[0056] According to the quality and molecular weight of the drug, DMSO was used to make a 10mM mother solution, and after shaking, it was found that it could be dissolved without precipitation. The two-fold dilution assay was started from 200 μM, the upper limit of drug toxicity. At the beginning, the concentrations of the three drugs were set at 200, 100, and 50 μM, respectively, the initial concentration of Porphyromonas gingivalis was 10^7 / mL, and the initial concentration of Fusobacterium nuclea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com