Function polyamide monomer, function polyamide and preparation methods
A technology of polyamide monomer and polyamide, which is applied in the field of functional polyamide and its preparation, and functional polyamide monomer, which can solve the problems of poor mechanical properties of elastomers and unsatisfactory mechanical properties, and achieve the effect of excellent aggregated structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0041] Embodiment 1-1 functional polyamide monomer 1 (R 1 For the preparation of -OH)
[0042] Take 100 g of methyl undecylenate and add 1 g of 1,3-diamino-propanol into 4 ml of tetrahydrofuran. After passing argon for half an hour, place it in a 40°C oil bath, add 10ml of sodium methoxide, the solution becomes clear, and react for 20 hours under the reaction conditions of 40°C. The specific reaction equation is as follows:
[0043]
[0044] Finally, after purification, a white powdery solid is obtained, and the white powdery solid is a functional polyamide monomer whose side group R1 is -OH, also called a hydroxyl amide monomer.
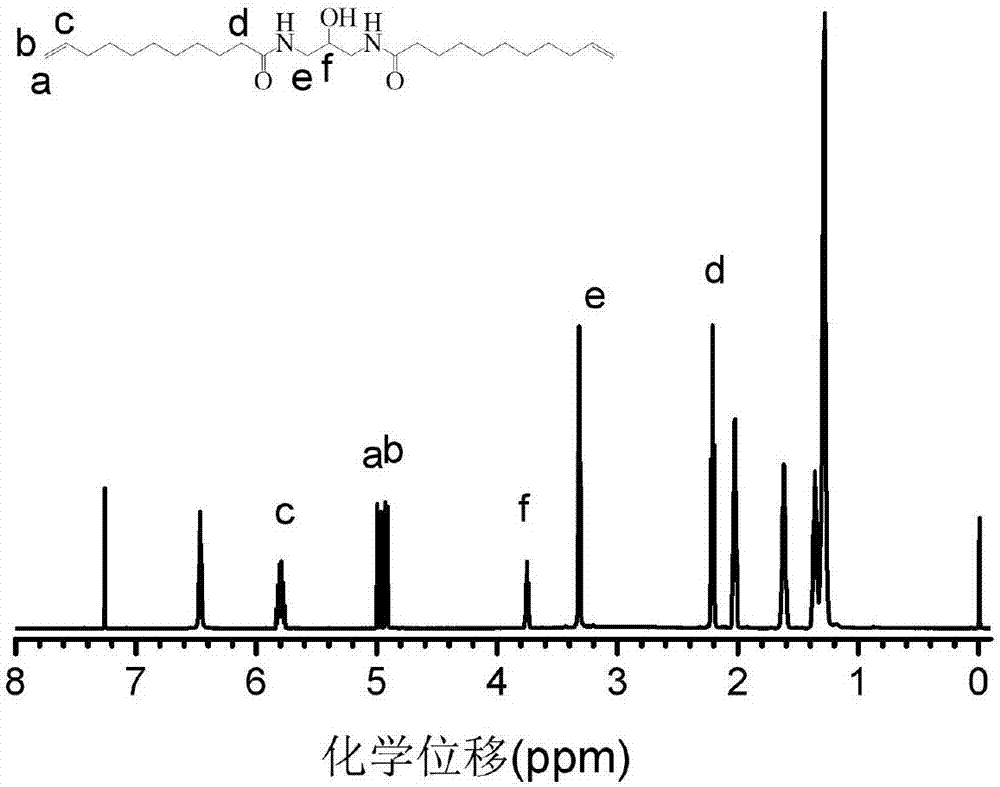
[0045] The 1H NMR spectrum of the functional polyamide monomer 1 prepared in this embodiment is as follows figure 1 As shown, the purity of the obtained functional polyamide monomer 1 is very high. From figure 1 Peak e at 3.38ppm and peak f at 3.8 can be seen that 1,3-diamino-propanol has been successfully integrated into the monomer, and the...
Embodiment 1-2
[0046] Embodiment 1-2 functional polyamide monomer 1 (R 1 For the preparation of -OH)
[0047]1 weight part of undecylenic acid methyl ester and 1 weight part of 1,3-diamino-propanol were added to 10 weight parts of tetrahydrofuran. After passing argon for half an hour, place it in an oil bath at 40°C, add 10 -4 After parts by weight of sodium methoxide, the solution becomes clear, reacted for 2 hours under the reaction condition of 40° C., and finally obtained a white powdery solid through recrystallization.
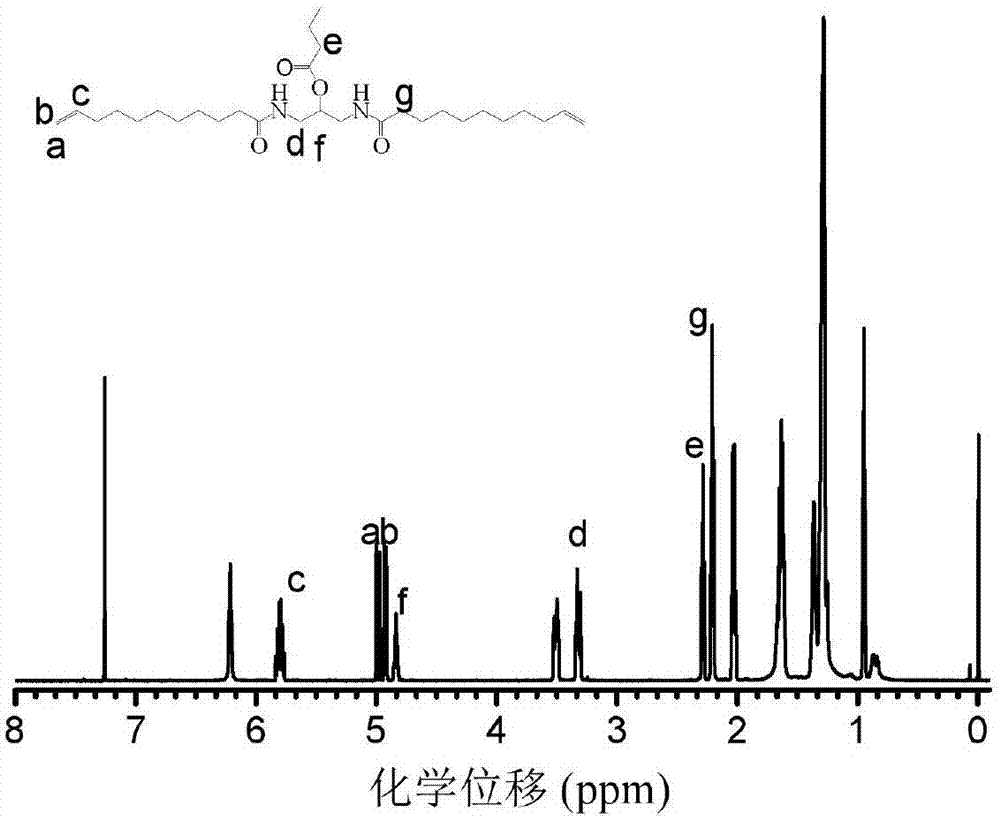
[0048] The 1H NMR spectrum of the functional polyamide monomer 1 prepared in this example also shows a high purity.
Embodiment 1-3
[0049] Embodiment 1-3 functional polyamide monomer 1 (R 1 For the preparation of -OH)
[0050] Take 1000 parts by weight of undecylenic acid methyl ester, and add 10 parts by weight of 1,3-diamino-propanol into 1000 parts by weight of tetrahydrofuran. After passing argon for half an hour, place it in an oil bath at 40°C, add 10 parts by weight of sodium methoxide, the solution becomes clear, react for 36 hours at 120°C, and finally obtain a white powdery solid through recrystallization.
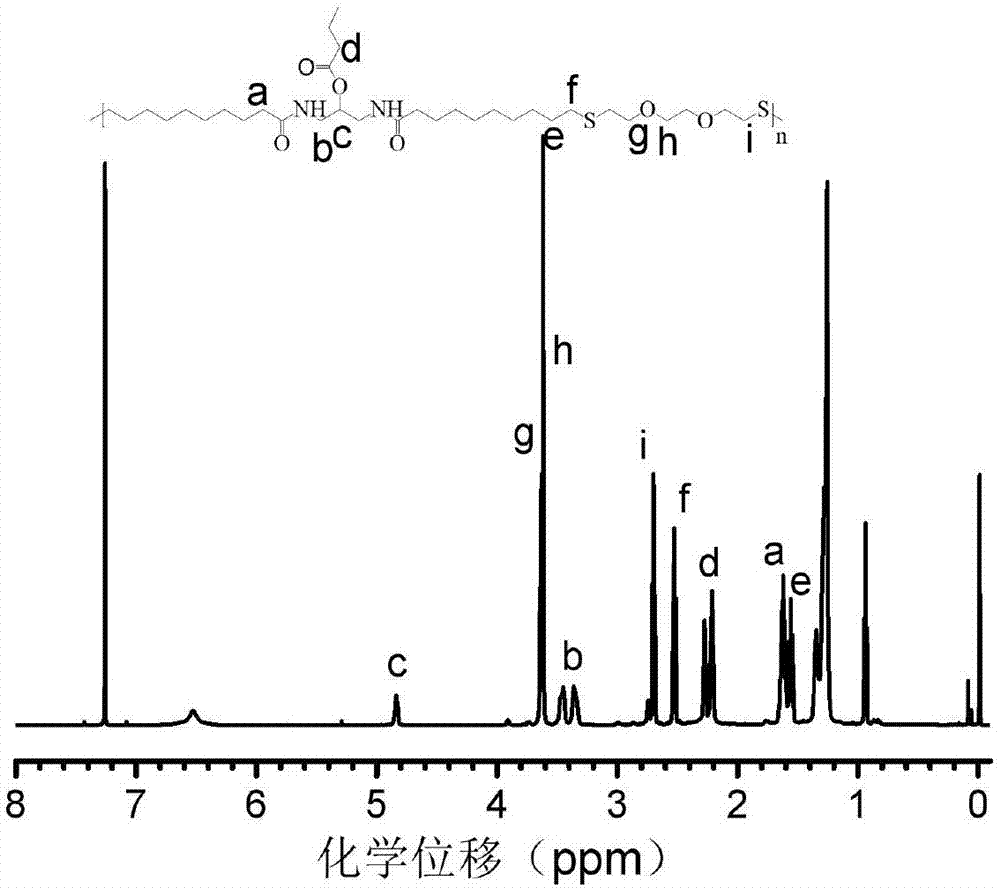
[0051] The 1H NMR spectrum of the functional polyamide monomer 1 prepared in this example also shows a high purity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com