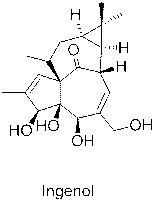
Quick identification method for ingenol
A fast technology for ingenol, applied in the field of rapid identification of ingenol by high-performance thin-layer chromatography, can solve problems such as complex structure, high detection cost, and long liquid chromatography analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 1 mg of ingenol reference substance in a volumetric flask, add 1 mL of methanol to dissolve it completely, and use it as the reference substance solution. In addition, get capers ethanol extract and reclaim the solvent upper layer, adjust the pH value to 12-14, hydrolyze at room temperature for 0.5h, and make the test solution according to [0009]; draw the above-mentioned reference solution and the test solution according to the thin-layer chromatography experiment 5 uL of each product solution was spotted on the same GF254 silica gel plate, developed (the mixture of petroleum ether and ethyl acetate was used as the developing solvent, the volume ratio of petroleum ether:ethyl acetate=3:7), taken out and dried, Spray with 5% vanillin-concentrated sulfuric acid solution (vanillin 5g, 10% sulfuric acid ethanol solution 100 mL)
[0026] For color development, heat until the spots are clear, and observe at 365nm; in the thin-layer chromatography of the reference substa...
Embodiment 2
[0028] Get caperia caperi ethanol extract and reclaim the upper layer of solvent and adjust the pH value to 12-14, room temperature hydrolysis 1h, be made into need testing solution according to [0009]; draw each 5 uL of above-mentioned need testing solution according to thin-layer chromatography experiment, and The reference solution was spotted on the same GF254 silica gel plate, developed (the mixture of petroleum ether and ethyl acetate was used as the developing solvent, the volume ratio of petroleum ether: ethyl acetate = 3:7), taken out and dried, and sprayed with 5% Vanillin-concentrated sulfuric acid solution (vanillin 5g, 10% sulfuric acid ethanol solution 100 mL) develops color, heats until the spots are clear, and observes at 365nm; Light blue, other spots appear orange, cyan or pink, see details figure 1 Point 3 in .
Embodiment 3
[0030] Get capers ethanol extract and reclaim solvent upper layer to adjust pH value to 12-14, room temperature hydrolysis 2h, be made into need testing solution according to [0009]; Draw each 5 uL of above-mentioned need testing solution according to thin-layer chromatography experiment, and The reference solution was spotted on the same GF254 silica gel plate, developed (the mixture of petroleum ether and ethyl acetate was used as the developing solvent, the volume ratio of petroleum ether: ethyl acetate = 3:7), taken out and dried, and sprayed with 5% Vanillin-concentrated sulfuric acid solution (vanillin 5g, 10% sulfuric acid ethanol solution 100 mL) develops color, heats until the spots are clear, and observes at 365nm; Light blue, other spots appear orange, cyan or pink, see details figure 1 Point 4 in .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com