Kit for detecting mutation of pathogenic genes of phenylketonuria
A phenylketonuria and kit technology, which is applied in the field of kits for detecting mutations in the pathogenic gene of phenylketonuria, can solve the problems of cumbersome, powerless prenatal diagnosis, and long time-consuming biochemical detection, and reduce the incidence rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
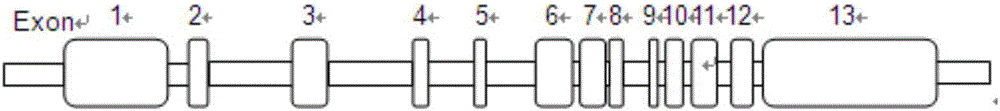
[0046] A kit for detecting mutations in the phenylketonuria pathogenic gene PAH for one person, including the following components: (1) 13 pairs of primers for PCR amplification and sequencing of exons 1 to 13 of the PAH gene, each primer 1OD, primer See Table 1 for the sequence;
[0047] (2) PCR amplification reagent: 7.5 μL of Taq enzyme mixture (dNTP, 10×PCR reaction buffer, MgCl 2 );
[0048] (3) PCR product purification reagent: 1.6 μL SAP enzyme mixture (SAP enzyme, ExoI enzyme, deionized water);
[0049] (3) Sequencing reagents: 2.2 μL BigDye mix (Bigdye, 5×seq), 2.5 μL EDTA, 30 μL ethanol solution (100%), 100 μL ethanol solution (70%), 8 μL Hi-Di.
[0050] This kit is stored at -20°C, and repeated freezing and thawing should be avoided as much as possible.
Embodiment 2
[0052] The steps for using a kit for detecting whether a mutation occurs in the phenylketonuria pathogenic gene PAH for one person include:
[0053] (1) extract the genomic DNA of the sample;
[0054] (2) PAH gene PCR amplification
[0055] For the exon 1-13 region of PAH gene, primers were designed according to Table 2 for PCR amplification. Each reaction system has a total volume of 16u 1, including 7.5 μL of Taq enzyme mixture (dNTP, 10×PCR reaction buffer, MgCl 2 ), deionized water 5.5 μL, primer pair (10uM) 1 μL, genomic DNA (100ng / ul) 1ul. The reaction conditions are 95°C for 15 minutes, 14 cycles (-0.5°C / cycle) of 94°C for 30 seconds, 63°C for 30 seconds, and 72°C for 45 seconds; then 30 cycles of 94°C for 30 seconds and 56°C for 30 seconds , 72°C for 45 seconds, and finally 72°C for 10 minutes.
[0056] (3) Purification of PCR products
[0057] Each reaction has a total volume of 5.6 μL. 4 μL of PCR product was added, and then 1.6 μL of SAP enzyme mixture (SAP en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com