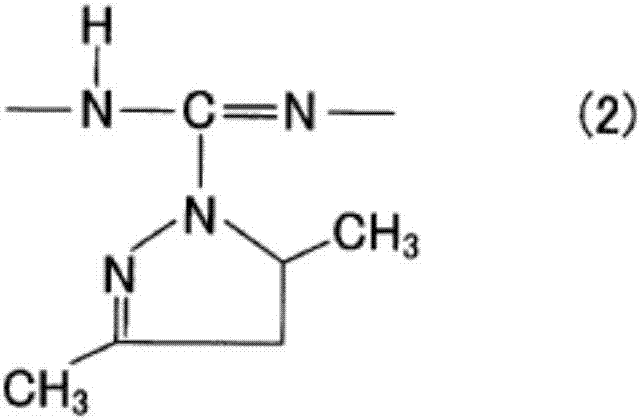
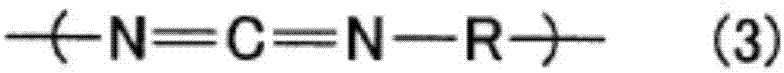Modified polycarbodiimide compound, curing agent, and thermosetting resin composition
一种聚碳化二亚胺、热固化性的技术,应用在聚脲/聚氨酯涂料、涂层等方向,能够解决溶解性低、长期保存困难等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] (Method for producing polycarbodiimide compound)
[0040]The polycarbodiimide compound used in the modified polycarbodiimide compound of the present invention can be produced by various methods using a diisocyanate compound as a raw material. For example, a method for preparing isocyanate-terminated polycarbodiimide by decarbonation condensation reaction accompanied by decarbonation of an aromatic diisocyanate compound (US Patent No. 2941956 specification or Japanese Patent Publication No. 47-33279, J. Org. Chem, 28, 2069-2075 (1963), Chemical Review 1981, Vol.81, No.4, p619-621, etc.).
[0041] The decarboxylation condensation reaction of the above-mentioned aromatic diisocyanate compound is performed in the presence of a carbodiimidization catalyst. Examples of the carbodiimidization catalyst include 1-phenyl-2-phosphole-1-oxide, 3-methyl-1-phenyl-2-phosphole-1 -oxide, 1-ethyl-2-phosphole-1-oxide, 3-methyl-2-phosphole-1-oxide and their 3-phosphole iso Constructed p...
Embodiment
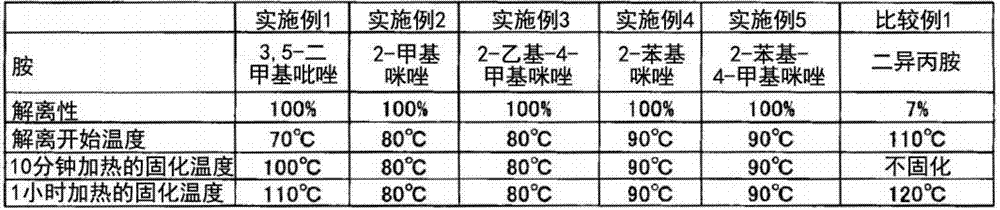
[0061] Hereinafter, although an Example and a comparative example are given and this invention is demonstrated more concretely, this invention is not limited to these. In addition, the following "%" is based on mass unless otherwise stated.
[0062] [Production of modified polycarbodiimide compounds]
[0063] The modified polycarbodiimide compounds of Examples and Comparative Examples were produced as follows.
Synthetic example 1
[0065] 100 parts by mass of toluene diisocyanate (composition: 80% of 2,4-toluene diisocyanate, 20% of 2,6-toluene diisocyanate), 71.8 parts by mass of polyalkylene carbonate diol (Asahi Kasei Chemical ( Co., Ltd., DURANOL T-5651, molecular weight 1000), 17.1 parts by mass of phenylisocyanate, 245 parts by mass of cyclohexanone, and 1.0 parts by mass of a carbodiimidization catalyst (3-methyl-1-phenyl- 2-phosphole-1-oxide) into a reaction vessel with a reflux tube and a stirrer, and stirred at 100° C. for 3 hours under a nitrogen stream, and confirmed that the wavelength was 2270 cm by infrared absorption (IR) spectroscopy. -1 The absorption peaks derived from the isocyanate group before and after almost disappeared, and the wavelength 2150cm was confirmed at the same time -1 The polycarbodiimide compound of Synthesis Example 1 was obtained according to the absorption peaks derived from the carbodiimide group before and after.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com