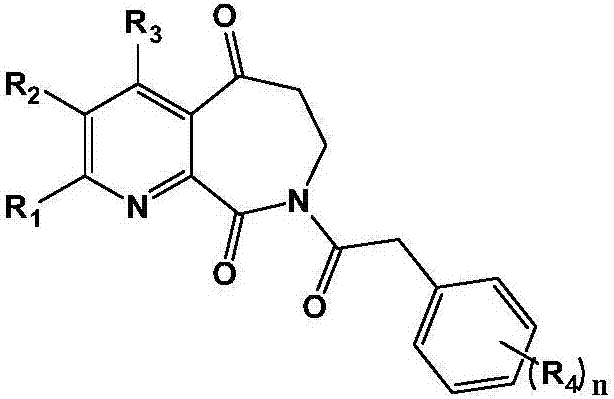
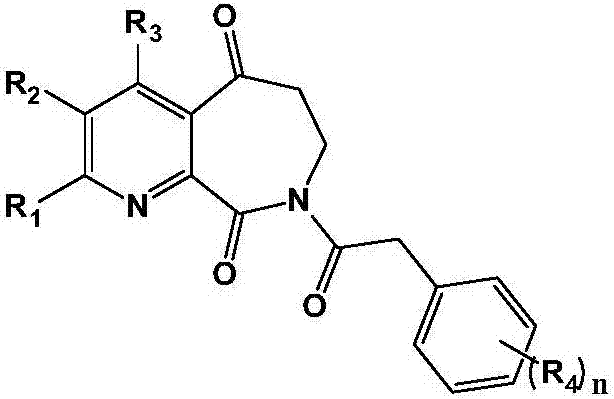
Medicine for treating liver cirrhosis and synthesis method thereof
A compound and pharmaceutical technology, applied in the field of drugs for the treatment of liver cirrhosis and its synthesis, can solve the problems of failure to achieve the expected effect, complex formation mechanism of liver fibrosis, etc., achieve prevention and treatment of liver fibrosis and liver cirrhosis, and inhibit liver fibrosis Effects of chemicalization and liver damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]Example 1: 8-(2-phenylacetyl)-7,8-dihydro-5H-pyridin[2,3-c]azepine-5,9(6H)-dione (AZE-1) Synthesis
[0059]
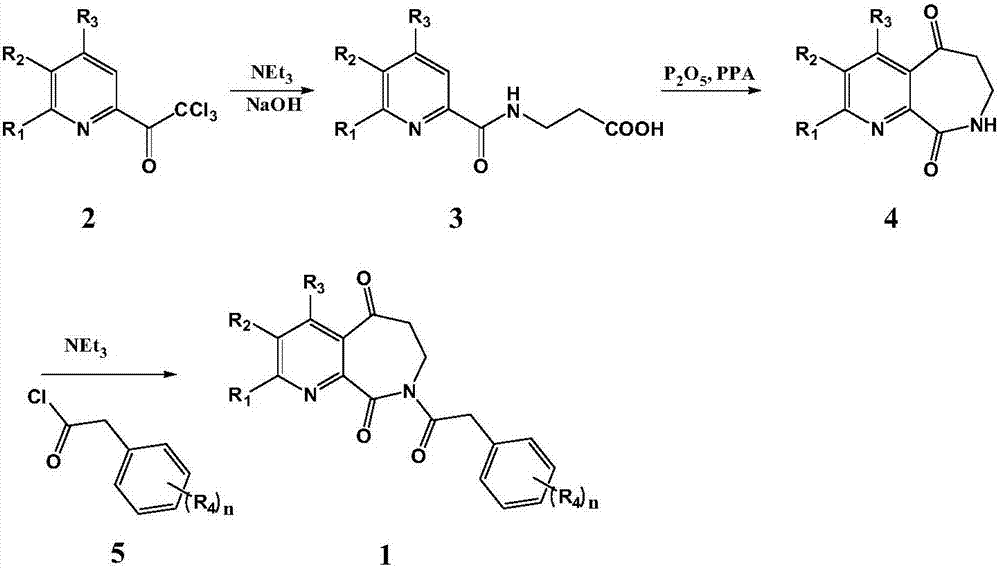
[0060] Step 1: In 150ml of anhydrous acetonitrile solution containing 8.9g 2,2,2-trichloro-1-(pyridin-2-yl)ethanone, add 7.38g β-alanine ethyl ester hydrochloride and 8ml tris Ethylamine, stirred at room temperature for about 8 hours, until the conversion of the raw materials was complete, the solvent was removed by rotary evaporation, and then 40mL of 2N NaOH solution was added, stirred at room temperature for 10 hours, the reaction solution was extracted with ethyl acetate, and the organic phase was extracted with water, 1N Wash with HCl solution and saturated brine respectively, and wash with anhydrous Na 2 SO 4 After drying, the solvent was removed by rotary evaporation under reduced pressure to obtain compound 3-(pyridinecarboxamide) propionic acid (6.9 g, yield 89%). ESI-MS: 195.07[M+H] +
[0061] Step 2: 9.80g P 2 o 5 Stir with 103.2g of PPA at 1...
Embodiment 2
[0067] Example 2: 8-(2-(4-methoxyphenyl)acetyl)-7,8-dihydro-5H-pyridin[2,3-c]azepine-5,9(6H)- Synthesis of diketone (AZE-2)
[0068]
[0069] According to the method of Example 1, except that phenylacetic acid was replaced by 4-methoxyphenylacetic acid, the title compound was obtained as a white solid, and the total yield of the three steps was 43%.
[0070] Elemental analysis: theoretical value / measured value, C(66.66 / 66.78), H(4.97 / 4.82), N(8.64 / 8.53), O(19.73 / 19.87)
[0071] ESI-MS: 325.11[M+H] +
[0072] 1 H NMR (400MHz, CDCl 3 )δ9.12(d,1H),8.74(d,1H),8.13(q,1H),7.13(s,2H),6.83(s,2H),3.93(s,2H),3.82(s,3H ), 3.72(t,2H), 2.63(t,2H).
[0073] According to a method similar to Example 1, the following compounds were synthesized by changing the raw materials used:
[0074]
[0075]
[0076] Next, the pharmacological effects of representative compounds are explained in detail through pharmacodynamic experiment examples.
experiment example 1
[0077] Drug efficacy experiment example 1: Evaluation of the target compound on inhibiting TGF-β production
[0078] In order to observe whether the compounds of the present invention can directly inhibit the production of TGF-β (which is overexpressed in macrophages) and evaluate the relevant molecular pharmacological mechanism, RAW264.7 macrophages were used for testing. When the compounds AZE-1 to AZE-6 of the present invention are directly added to RAW264.7 macrophages (which have increased the expression of TGF-β), taking AZE-1, AZE-3 and AZE-6 as examples, the test results are as follows :
[0079] Table 1: Inhibition of TGF-β expression in macrophages by target compounds
[0080]
[0081] The test results show that the compound of the present invention can inhibit the expression of TGF-β in a dose-dependent manner, indicating that the compound of the present invention can be used as an anti-fibrosis and anti-cirrhosis drug in hepatic macrophages of the liver by inhi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com