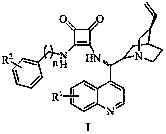
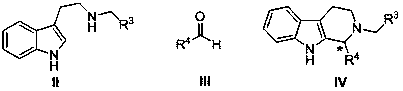
Application and application method of cinchona alkaloid squaramide derivative as catalyst in asymmetric P-S reaction
A technology of cinchona square amide and base square amide, which is applied to the application and application field of cinchona square amide derivatives as catalysts in asymmetric P‑S reactions, and can solve the constraints of large-scale application of catalysts and complex catalyst preparation processes , modifying groups cannot be removed, etc., to achieve good application value, rich variety, high yield and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
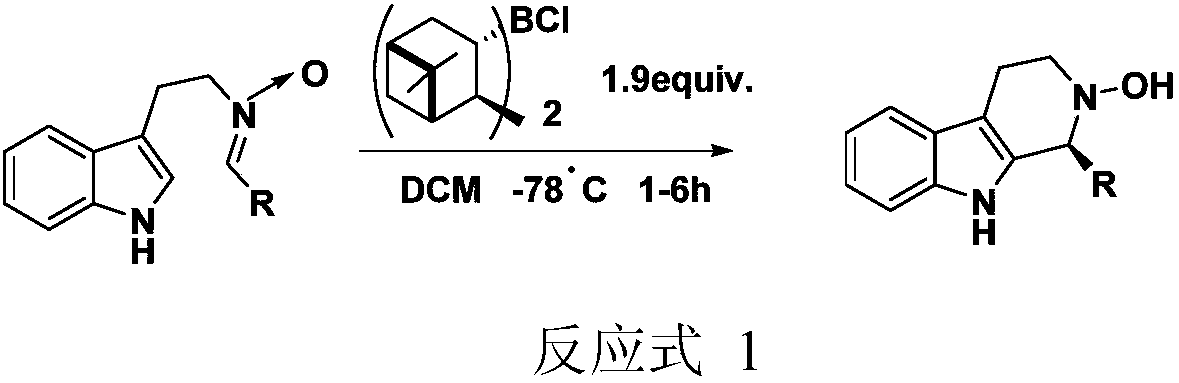
[0034] Example 1: Synthesis of (S)-2-benzyl-1-phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]-indole.
[0035] In a 25mL single-neck flask, add cinchonaine squarine derivative catalyst Ia (0.5mmol, 0.32g), benzyltryptamine (5mmol, 1.25g), benzaldehyde (7.5mmol, 0.80g), toluene (5mL), 100 After reacting at ℃ for 6h, the reaction solution was concentrated and separated by column chromatography to obtain (S)-2-benzyl-1-phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4- b]-Indole 1.59g, white solid, the yield was 94%, and the ee value was 89%.
[0036]
[0037] 1 H NMR(500MHz, CDCl 3 )δ2.65-2.70(m,1H), 2.79-2.83(m,1H), 2.90-2.96(m,1H), 3.23-3.27(m,1H), 3.37(d,J=13.5Hz,1H) ,3.90(d,J=13.5Hz,1H),4.65(s,1H),7.10-7.14(m,2H),7.18-7.19(m,1H),7.25-7.28(m,1H),7.32-7.35 (m,3H),7.36-7.39(m,4H),7.47(m,2H),7.53-7.54(m,1H). 13 C NMR(125MHz, CDCl 3 )δ141.46,139.57,136.29,134.86,129.02(2C),128.76(2C),128.70(2C),128.23(2C),128.08,127.21,126.92,121.49,119.35,118.30,110.78,108.95,64.60,58.33,48.35 ,21.18.HPLC[Daicel...
Embodiment 2
[0038] Example 2: Synthesis of (S)-2-benzyl-1-(4-fluorophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]-indole .
[0039] In a 25mL single-neck flask, add cinchonaine squaraamide derivative catalyst Ib (1.5mmol, 0.94g), benzyltryptamine (5mmol, 1.25g), 4-fluorobenzaldehyde (7.5mmol, 0.93g), dichloromethane (5mL), reacted at 40℃ for 18h, the reaction solution was concentrated and separated by column chromatography to obtain (S)-2-benzyl-1-(4-fluorophenyl)-2,3,4,9-tetrahydro -1H-pyrido[3,4-b]-indole 1.71g, white solid, the yield was 96%, and the ee value was 84%.
[0040]
[0041] 1 H NMR(500MHz, CDCl 3 )δ2.68-2.73(m,1H),2.81-2.86(m,1H),2.90-2.96(m,1H),3.22-3.27(m,1H), 3.39(d,J=8.5Hz,1H) ,3.89(d,J=8.5Hz,1H),4.67(s,1H),7.04-7.08(m,2H),7.10-7.15(m,2H),7.20-7.23(m,1H),7.26-7.28 (m, 1H), 7.32-7.36 (m, 4H), 7.39-7.43 (m, 2H), 7.53 (d, J = 6.0 Hz, 1H). 13 C NMR(125MHz, CDCl 3 )δ163.50,161.54,139.39,137.24,136.31,134.47,130.59,130.53,128.68,128.29(2C),127.16,127.02,121.67,119.47,118.37,115.70...
Embodiment 3
[0042] Example 3: Synthesis of (S)-2-(1-naphthylmethyl)-1-phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]-indole .
[0043] In a 25mL single-necked flask, add cinchonaine squarine derivative catalyst Ic (1mmol, 0.59g), 1-naphthyltryptamine (5mmol, 1.51g), benzaldehyde (5mmol, 0.53g), DMF (10mL), 25 After reacting at ℃ for 70h, the reaction solution was concentrated and separated by column chromatography to obtain (S)-2-(1-naphthylmethyl)-1-phenyl-2,3,4,9-tetrahydro-1H-pyrido [3,4-b]-Indole 1.49g, white solid, the yield was 77%, and the ee value was 87%.
[0044]
[0045] 1 H NMR(500MHz, CDCl 3 )δ2.69-2.74(m,1H), 2.80-2.91(m,2H), 3.22-3.26(m,1H), 3.83(d,J=13.5Hz,1H), 4.30(d,J=13.0Hz ,1H),4.73(s,1H),7.10-7.16(m,2H),7.21-7.22(m,1H),7.32-7.35(m,2H),7.36-7.37(m,1H),7.38-7.41 (m,1H),7.42-7.48(m,4H),7.53-7.55(m,1H),7.58(d,J=8.4Hz,1H),7.77(d,J=10.2Hz,1H),7.84( d, J = 9.6 Hz, 1H), 8.04 (d, J = 10.2 Hz, 1H). 13 C NMR(125MHz, CDCl 3 )δ141.32,136.31,134.97,134.68,133.80,132.38,129.39,128.60,128...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com