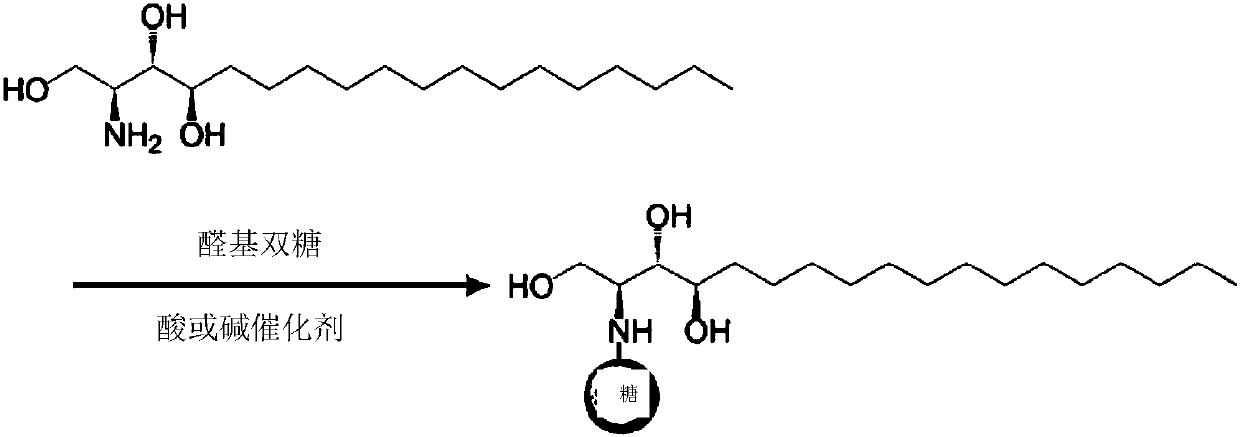
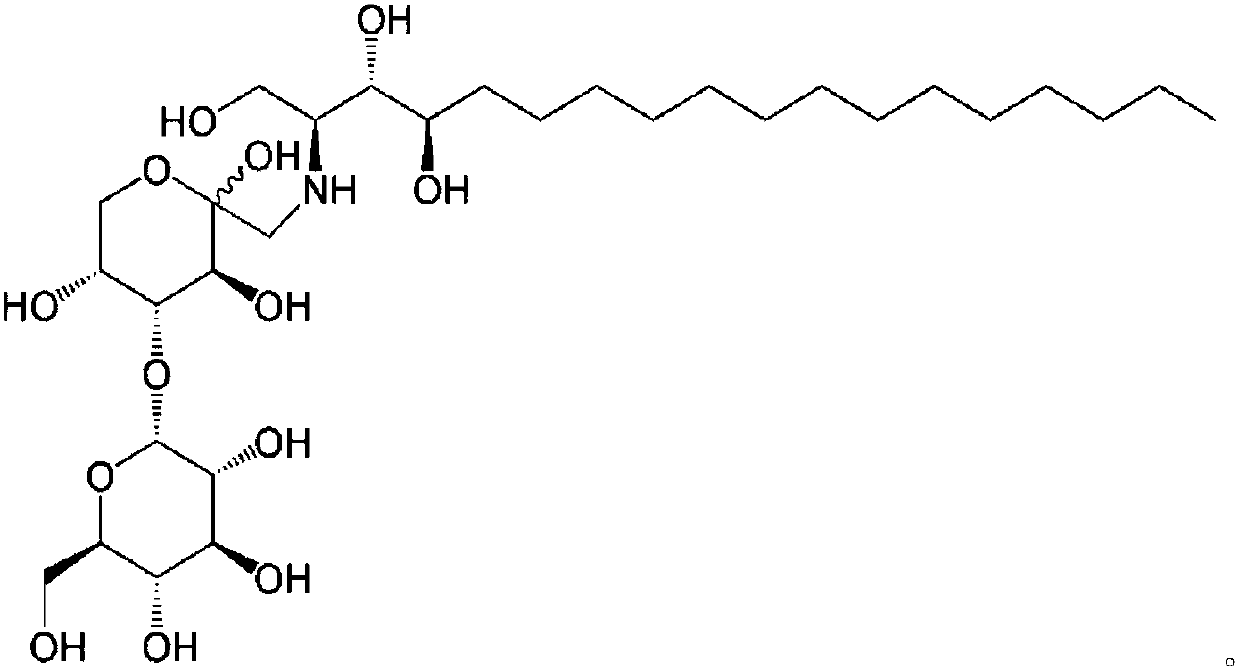
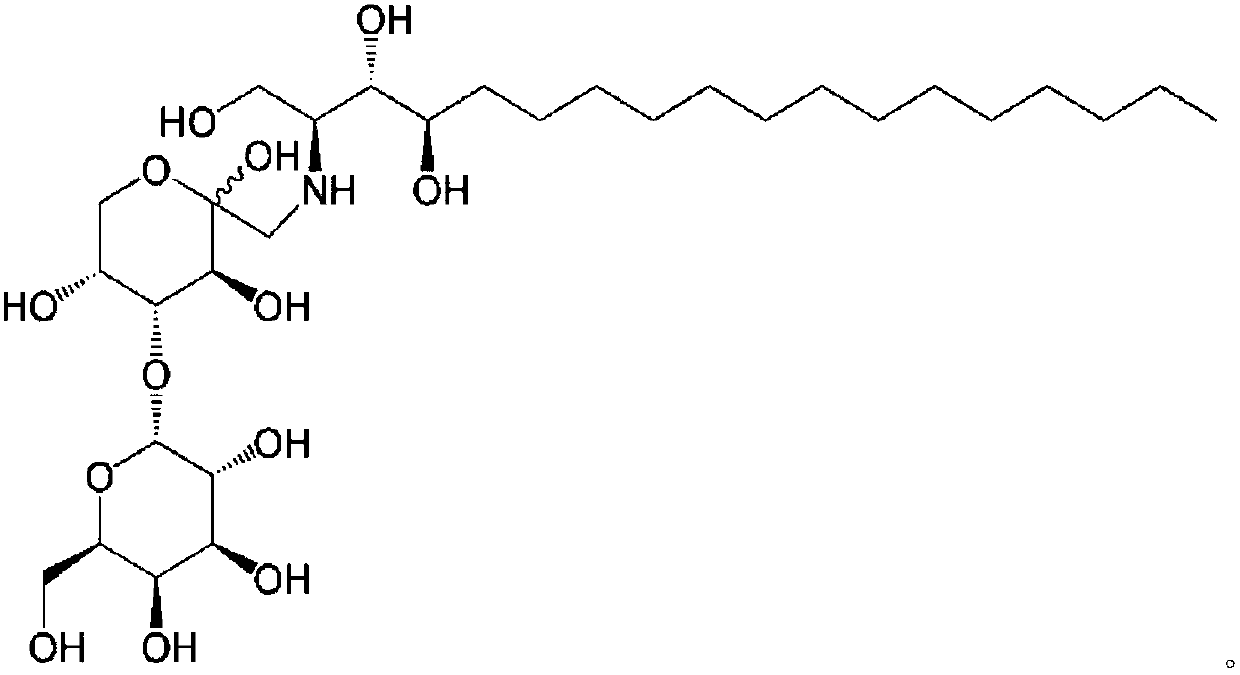
Phytospingosine derivative and composition containing same
A technology of phytosphingosine and derivatives, which is applied in the direction of drug combination, sugar derivatives, sugar derivatives, etc., can solve the problems of prone to sedimentation, achieve the effect of improving stability and improving antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of phytosphingosine derivative
[0048] Put 3.17 g of phytosphingosine, 3.78 g of maltose monohydrate and 30 μL of acetic acid into 40 mL of methanol for circulation, and the reaction time is 12 hours. After the reaction, the solution was cooled at room temperature, and the product was precipitated after adding acetone. The precipitated phytosphingosine derivative was filtered and then dried.
[0049] 1 H NMR (300MHz,D 2 O)δ:0.89(3H),1.10-1.50(26H),2.90-3.20(4H),3.25-4.20(14H),4.66&5.20-5.45(1H)
Embodiment 2
[0050] Embodiment 2: the synthesis of phytosphingosine derivative
[0051] Except that lactose monohydrate (Lactose monohydrate) was used instead of maltose monohydrate, others were prepared by the same method as in Example 1 to prepare phytosphingosine.
[0052] 1 H NMR (300MHz,D 2 O)δ:0.89(3H),1.10-1.50(26H),2.90-3.20(4H),3.25-4.20(14H),4.66&5.20-5.45(1H)
experiment example 1
[0053] Experimental Example 1: Solubility Test of Phytosphingosine Derivatives
[0054] Dissolve the phytosphingosine derivatives obtained in Example 1 and Example 2 in distilled water at 50-60°C so that the weight of the overall aqueous solution is 0.1% by weight and 1.0% by weight for comparison, and then put it at room temperature, observe the following The precipitation results at different times. As a comparative example, using the same phytosphingosine to carry out the same experiment, the results are as follows figure 1 shown.
[0055] Table 1
[0056]
[0057] ○: Not yet precipitated
[0058] △: Although precipitation is not visible to the naked eye, fine particles are generated.
[0059] x: Precipitation was seen with the naked eye.
[0060] As shown in Table 1 above, compared with the phytosphingosine of the comparative example, the solubility in water of the phytosphingosine derivative of the present invention is increased, and it will not be precipitated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com