Application of isobavachalcone in preparation of therapeutic drug for acute myeloid leukemia (AML)
A technology of acute myeloid cells and psoralen B, which is applied in the fields of chemical industry and medicine, and can solve the problems that the application of psoralen B has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Expression and Purification of Human Recombinant hDHODH
[0037] Transform E.coli BL21(DE3) competent cells with the recombinant plasmid pET-19b-hDHODH with correct sequencing, spread it on the LB plate containing ampicillin and culture it, pick a single clone and inoculate it in a 2× Cultivate overnight in YT medium on a shaker at 37°C and 230 rpm. After overnight culture, the bacteria were inoculated in 8×1L TB medium containing 100g / mL ampicillin at a ratio of 1:200 and expanded at 37°C and 230rpm. When the OD value of the bacteria reaches 0.8-1, IPTG is added to the medium to make the final concentration of IPTG 0.5mM, and the expression of the fermentation broth is induced overnight at 16°C.
[0038] After overnight culture, the induced cells were collected by centrifugation at 4°C and 4000rpm, the cells were washed once with a buffer solution of 50mM HEPES pH8.0, 400mM NaCl, the cell pellet was collected by centrifugation, and the cells were stored at -...
Embodiment 2
[0041] Example 2 Screening of human recombinant hDHODH inhibitors
[0042] The purified hDHODH protein was diluted to 10 μM with an activity test solution, which was 50 mM HEPES pH 8.0, 150 mM KCl, 0.1% Triton X-100. Add Coenzyme Q 0 and DCIP, so that the final concentrations were 100 μM and 120 μM. After mixing well, put it into a 96-well plate, incubate at room temperature for 5 minutes, then add the substrate DHO to start the reaction, and the final concentration of DHO is 500 μM. Use a PE microplate reader to detect the absorbance at 600 nm, read it every 30 s for 6 min, and calculate the inhibition rate.
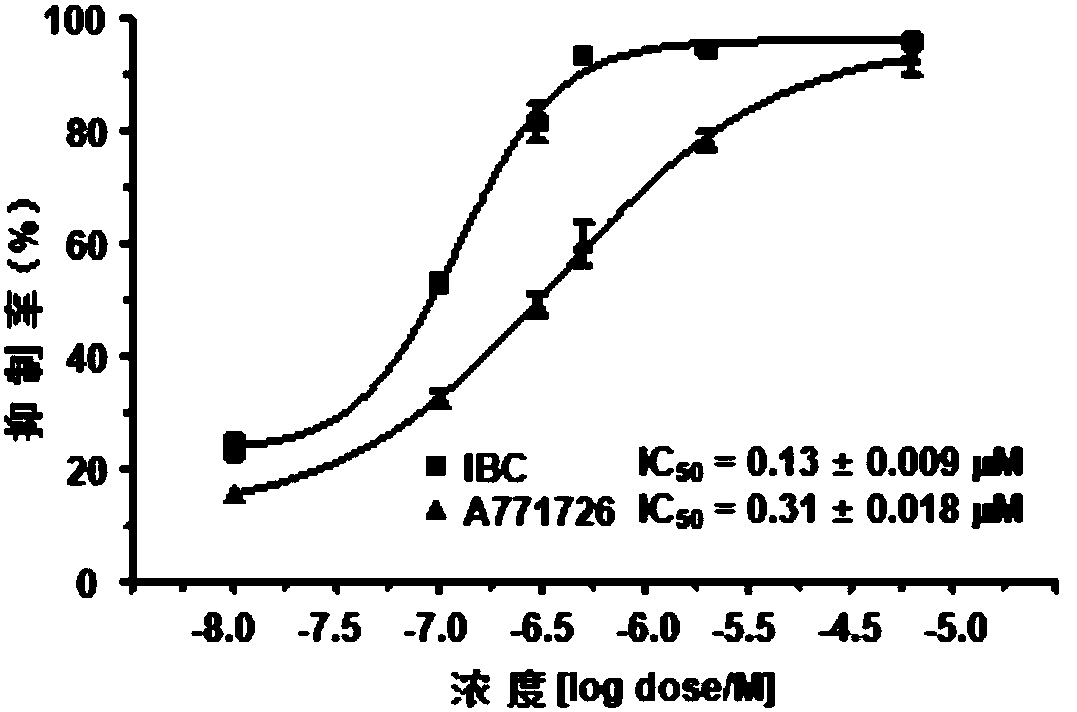
[0043] figure 2 It is the linear relationship between psoralen B and hDHODH activity, and psoralen B has strong inhibitory effect on hDHODH activity; figure 2 Among them, the abscissa is the psoralen B concentration (μM), and the ordinate is the enzyme activity inhibition rate Inhibition (%); the compounds are psoralen B and the positive control A771726 respective...
Embodiment 3
[0044] Example 3 Study on thermal stability of psoralen B to human recombinant hDHODH
[0045] The hDHODH protein was diluted to 5 μM with the test solution, and the activity test solution was 25 mM HEPES, 150 mM NaCl, pH7.5. Add the fluorescent dye Sypro orange to a final concentration of 5X. After mixing, add 40 μL per well into a 96-well plate, and then add the compound at a pre-set concentration for incubation. Use Bio-Rad RT-PCR to determine the protein Tm value.
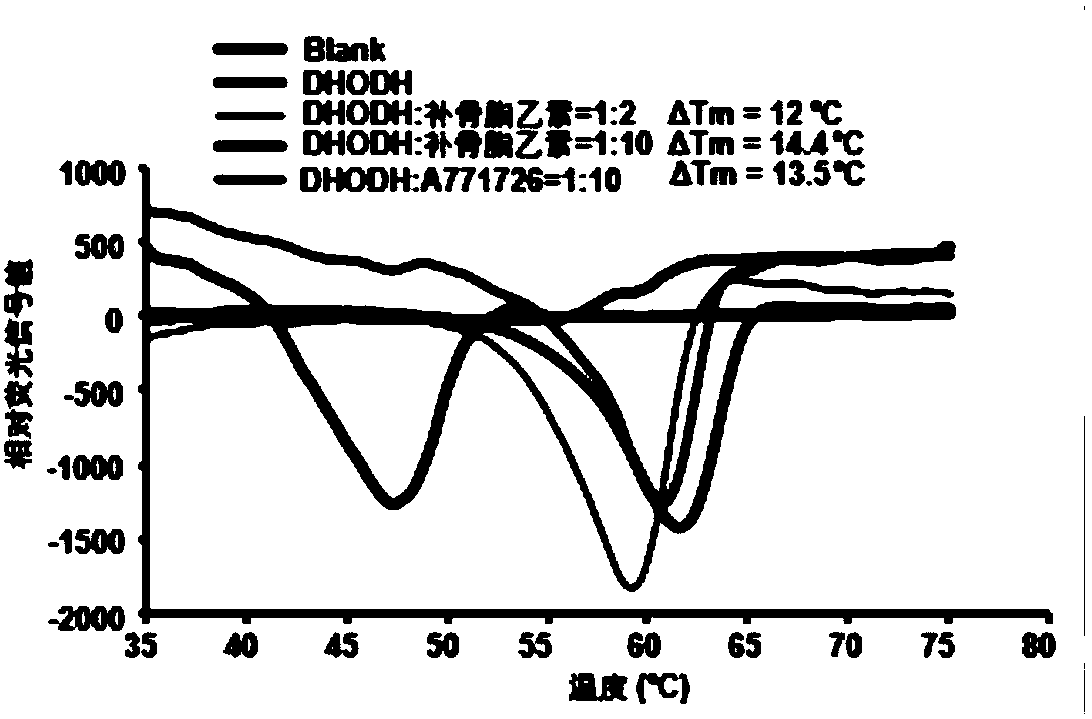
[0046] image 3 After incubating psoralen B and hDHODH protein at concentrations of 2:1 and 10:1, respectively, the melting temperature of the protein was measured. The results showed that psoralen B could significantly increase the melting temperature of hDHODH protein. Under the conditions of these two ratios, the dissolution temperature increased by 12°C and 14.4°C respectively, which indicated that psoralen B could directly combine with hDHODH protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com