Pharmaceutical composition for treating Alzheimer's disease, and preparation method of pharmaceutical composition
A technology for Alzheimer's disease and composition, which is applied in the field of medicine, can solve the problems of inability to delay the development of the disease, liver and kidney toxicity of western medicine, and insignificant curative effect, and achieve the effect of treating Alzheimer's disease, broad medical application prospects, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
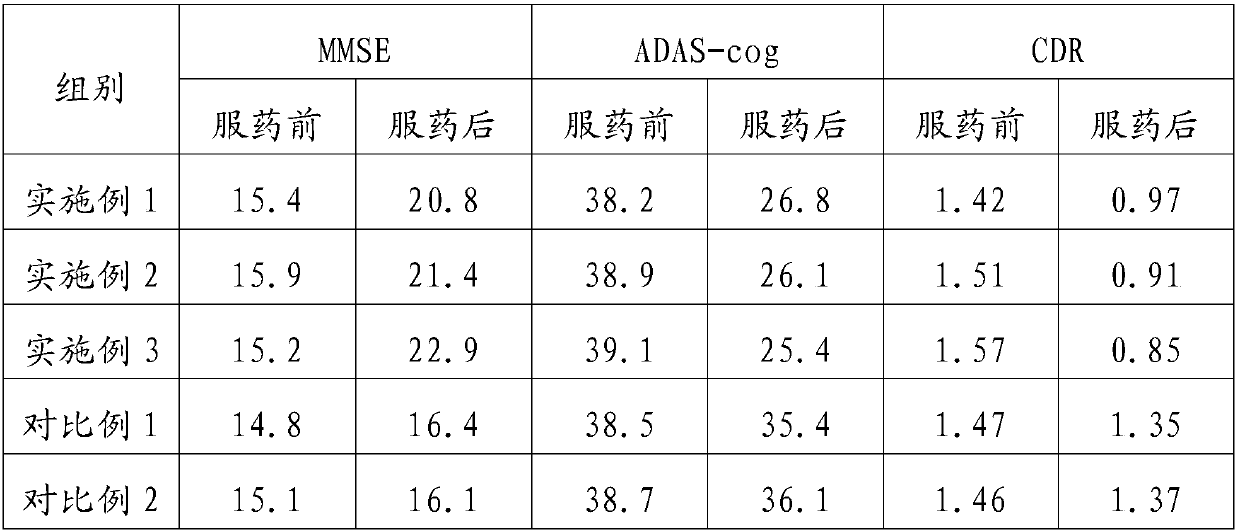
Embodiment 1
[0020] A preparation method for a pharmaceutical composition for treating senile dementia, comprising the steps of:
[0021] S1: Make 10 parts of Epimedium, 7 parts of Rehmannia glutinosa, 7 parts of Chinese yam, 8 parts of ginkgo biloba, 8 parts of safflower, 10 parts of earthworm, and 10 parts of Huhuanglian, add water to decoct 3 times, and filter , combine the filtrates, concentrate the filtrates under reduced pressure to 3 times the amount of raw materials, add ethanol with a mass concentration of 60-70%, stand still for 24-48h, remove the supernatant, concentrate and dry the precipitate under reduced pressure, and pulverize into fine powder for later use;
[0022] S2: Grinding 2 parts of nimodipine and 2 parts of donepezil hydrochloride into fine powder 2;
[0023] S3: Mix the fine powder 2 obtained in step S2 with the fine powder 1 obtained in step S1, add sodium carboxymethyl starch, dextrin, and magnesium stearate, mix evenly, granulate, pack into capsules, and make c...
Embodiment 2
[0025] A preparation method for a pharmaceutical composition for treating senile dementia, comprising the steps of:
[0026] S1: Make 20 parts of Epimedium, 14 parts of Rehmannia glutinosa, 14 parts of Chinese yam, 12 parts of ginkgo biloba, 12 parts of safflower, 12 parts of earthworm, and 12 parts of Huhuanglian, add water to decoct 3 times, and filter , combine the filtrates, concentrate the filtrates under reduced pressure to 3 times the amount of raw materials, add ethanol with a mass concentration of 60-70%, stand still for 24-48h, remove the supernatant, concentrate and dry the precipitate under reduced pressure, and pulverize into fine powder for later use;
[0027] S2: Grinding 3 parts of nimodipine and 3 parts of donepezil hydrochloride into fine powder 2;
[0028] S3: Mix the fine powder 2 obtained in step S2 with the fine powder 1 obtained in step S1, add sodium carboxymethyl starch, dextrin, and magnesium stearate, mix evenly, granulate, pack into capsules, and ma...
Embodiment 3
[0030] A preparation method for a pharmaceutical composition for treating senile dementia, comprising the steps of:
[0031] S1: Make 30 parts of epimedium, 20 parts of Rehmannia glutinosa, 20 parts of Chinese yam, 16 parts of ginkgo biloba, 16 parts of safflower, 15 parts of earthworm, and 15 parts of Huhuanglian, add water to decoct 3 times, and filter , combine the filtrates, concentrate the filtrates under reduced pressure to 3 times the amount of raw materials, add ethanol with a mass concentration of 60-70%, stand still for 24-48h, remove the supernatant, concentrate and dry the precipitate under reduced pressure, and pulverize into fine powder for later use;
[0032] S2: Grinding 4 parts of nimodipine and 6 parts of donepezil hydrochloride into fine powder 2;
[0033] S3: Mix the fine powder 2 obtained in step S2 with the fine powder 1 obtained in step S1, add sodium carboxymethyl starch, dextrin, and magnesium stearate, mix evenly, granulate, pack into capsules, and ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com