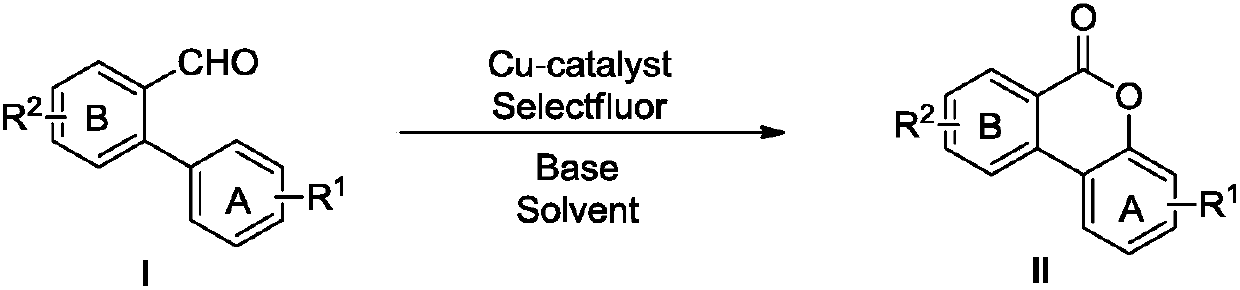
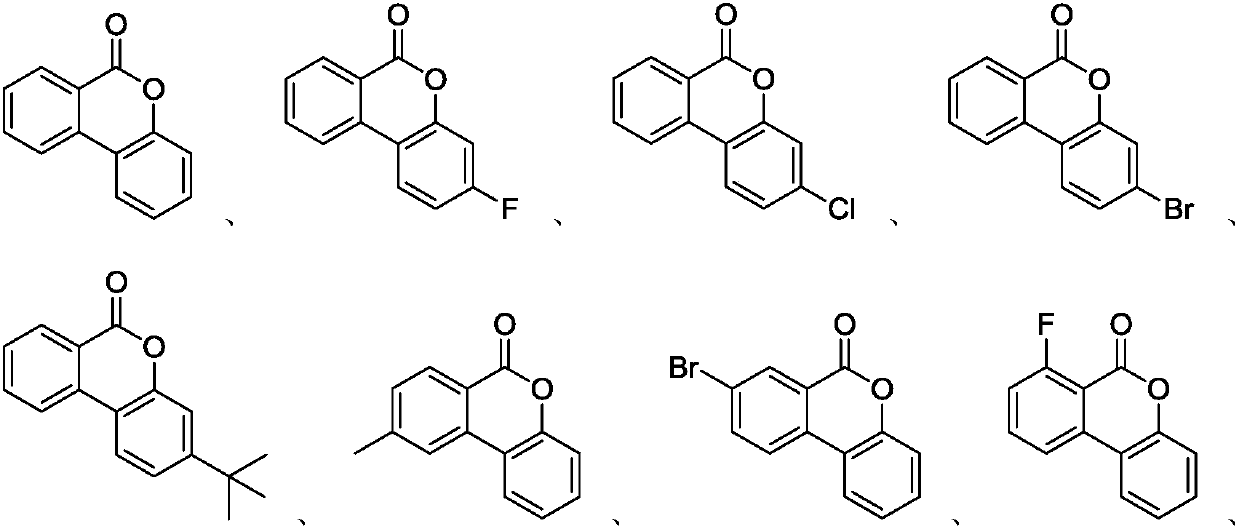
Method for synthesizing of isocoumarin compounds
A technology of isocoumarin and compounds, applied in the direction of organic chemistry, can solve the problems of expensive o-hydroxyphenylboronic acid, narrow application range, harsh reaction conditions, etc., and achieve the effect of simple reaction steps, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] 0.3mmol 2-phenylbenzaldehyde (54.6mg), 0.03mmol Cu powder (1.92mg), 0.6mmol Selectfluor (212.4mg) and 0.45mmol K 2 CO 3 (62.1mg) was added to a 15mL thick-walled pressure-resistant reaction tube, and then 3mL of acetonitrile was added as a solvent. Then, it was stirred magnetically at 25°C for 24 hours. After cooling to room temperature, 10 mL of water was added, and extracted with dichloromethane (3*10 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 After drying, the filtrate was taken by suction filtration to remove the solvent, and then separated by silica gel column chromatography, using petroleum ether / ethyl acetate = 30:1 as the eluent, collecting the eluate containing the target product and evaporating the solvent to obtain benzisocoumarin . The material was a white solid in 86% yield.
[0035] Characterization data: IR(KBr,cm -1 ):ν=1733,1602; 1 H NMR (500MHz, CDCl 3 ):δ8.43(dd,J 1 =7.5Hz,J 2 =1.0Hz,1H),8.15(d,J=8....
Embodiment 2
[0037]
[0038] 0.3mmol 2-phenylbenzaldehyde (54.6mg), 0.03mmol Cu powder (1.92mg), 0.45mmol Selectfluor (159.4mg) and 0.75mmol K 2 CO 3 (103.5 mg) was added to a 15 mL thick-walled pressure-resistant reaction tube, and then 3 mL of acetonitrile was added as a solvent. Then, it was magnetically stirred at 50° C. for 6 hours. After cooling to room temperature, 10 mL of water was added, extracted with dichloromethane (3*10 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 Dry, and then filter the filtrate to remove the solvent, then separate it by silica gel column chromatography, use petroleum ether / ethyl acetate = 30:1 as the eluent, collect the eluate containing the target product and evaporate the solvent to obtain benzoisocoumarin white. The material was a white solid in 76% yield.
[0039] Characterization data: IR(KBr,cm -1 ):ν=1733,1602; 1 H NMR (500MHz, CDCl 3 ):δ8.43(dd,J 1 =7.5Hz,J 2=1.0Hz,1H),8.15(d,J=8.5Hz,1H),8.09(dd,J 1 =8.0Hz...
Embodiment 3
[0041]
[0042] 0.3mmol 2-phenylbenzaldehyde (54.6mg), 0.015mmol Cu powder (0.96mg), 0.75mmol Selectfluor (265.7mg) and 0.9mmol K 2 CO 3 (124.2 mg) was added to a 15 mL thick-walled pressure-resistant reaction tube, and then 3 mL of acetonitrile was added as a solvent. Then, it was magnetically stirred at 0°C for 36 hours. After cooling to room temperature, 10 mL of water was added, and extracted with dichloromethane (3*10 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 Dry, and then filter the filtrate to remove the solvent, then separate it by silica gel column chromatography, use petroleum ether / ethyl acetate = 30:1 as the eluent, collect the eluate containing the target product and evaporate the solvent to obtain benzoisocoumarin white. The material was a white solid in 80% yield.
[0043] Characterization data: IR(KBr,cm -1 ):ν=1733,1602; 1 H NMR (500MHz, CDCl 3 ):δ8.43(dd,J 1 =7.5Hz,J 2 =1.0Hz,1H),8.15(d,J=8.5Hz,1H),8.09(dd,J 1 =8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com