Preparation method and application of tacrine-sinapic acid heterozygote
A technology of sinapinic acid and tacrine, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as limiting clinical application, and achieve strong inhibitory effect and obvious anti-oxidation activity, significant in vitro neuroprotective activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 intermediate 1
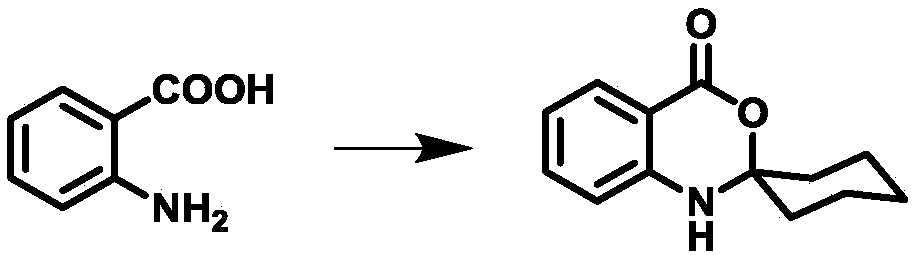
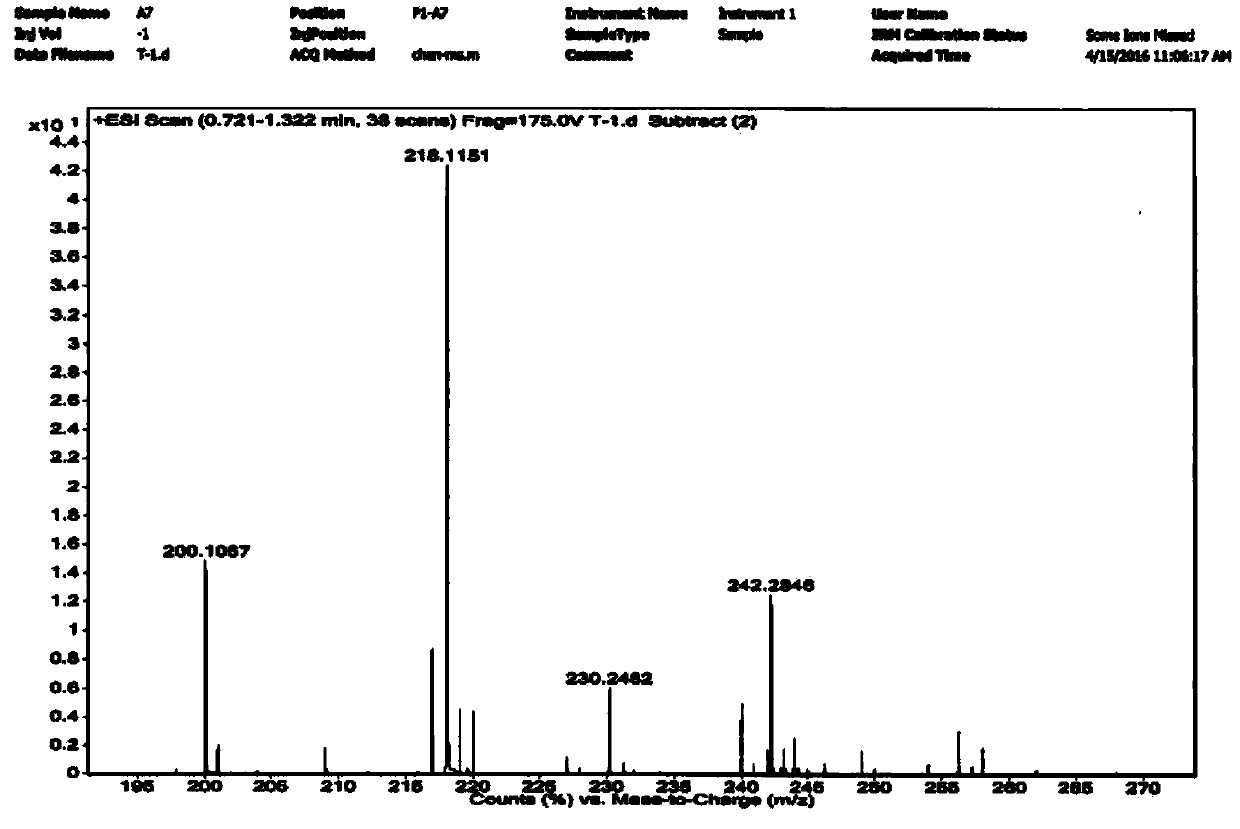
[0046] Weigh anthranilic acid (50.0g, 364mmol) and dissolve it in 100ml of toluene, add (45.3ml, 437mmol) cyclohexanone, reflux at 110.6°C for 2h, and remove about 6.8ml of water. The mixture was cooled to room temperature, and needle-like crystals precipitated out, which were further cooled to 0°C, filtered, and washed with toluene (50 mL) and ethanol (50 mL). Collect filter cake, dry in vacuum oven, obtain intermediate 1 white crystal 53.2g, yield 67% (synthetic route sees figure 1 , see the characterization map figure 2 ).
Embodiment 2
[0047] The synthesis of embodiment 2 intermediate 2
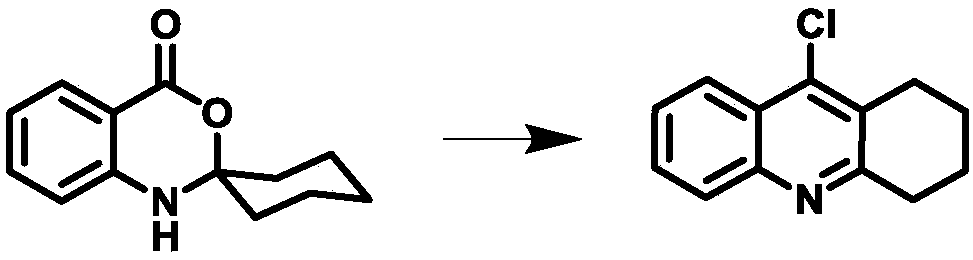
[0048] Intermediate 1 (67.0 g, 309 mmol) and phosphorus oxychloride (120 ml, 1.29 mol) were successively added into the three-necked flask, and the mixture was refluxed at 85° C. for 2 h. After cooling to room temperature, cold 54% KOH aqueous solution (540 g, 1000 ml) was added dropwise and stirred rapidly. After the addition was completed, a precipitate (yellow solid) was precipitated, and the pH of the supernatant was 10±1. Then add CH 2 Cl 2 (1500ml) to dissolve the solid and wash with CH 2 Cl 2 (3×1000ml) extract the aqueous layer, combine the organic phase, anhydrous MgSO 4 Dry, filter, reclaim solvent, obtain intermediate 2 yellow solid 60.0g, yield 90% (synthetic route sees image 3 ).
Embodiment 3
[0049] The synthesis of embodiment 3 aminotacrine
[0050] Intermediate 2 (30.0 g, 0.14 mol), hexamethylenediamine (54 ml, 0.42 mol) and 1.5 g KI were sequentially added to the three-necked flask, dissolved in 200 ml of n-pentanol, and refluxed at 140° C. for 18 h. The reaction solution was cooled to 0°C, the pH was adjusted to 1.0 with 6M hydrochloric acid, and the layers were separated. The aqueous layer was adjusted to pH 10.0 with cold KOH solution, extracted with dichloromethane (3×200ml), the organic phases were combined, washed three times with saturated NaCl solution, anhydrous NaCl 2 SO 4 Dry, filter and evaporate to dryness under reduced pressure. Purified by column chromatography (eluent: dichloromethane:methanol=50:1-10:1-5:1, 10ml triethylamine / 1000ml) to obtain 36.3g of aminotacrine light yellow oily liquid with a yield of 88% % (synthetic route sees Figure 4 , see the characterization map Figure 5-6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com