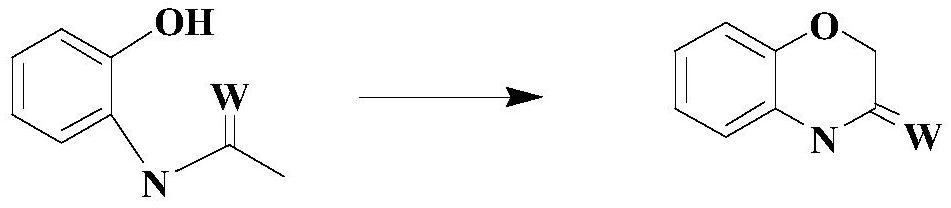
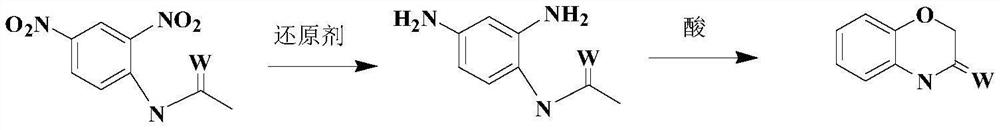
A kind of synthetic technique of 1,4 benzoxazinone compound
A technology of benzoxazinones and compounds, which is applied in the direction of organic chemistry, can solve the problems of complex synthesis methods of intermediates, high purity requirements of raw materials, air and light sensitivity, etc., so as to avoid instability and unsafety and low price , the effect of simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 6-amino-1,4-benzoxazin-3-(4H)-one
[0026]
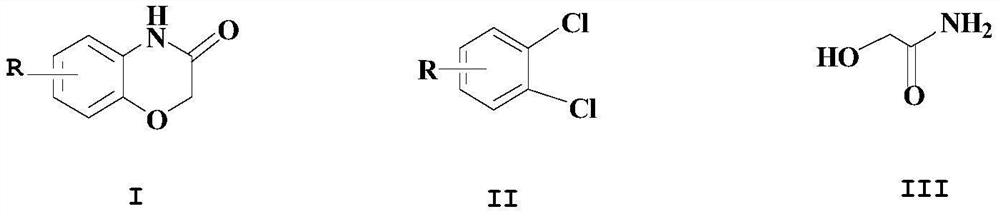
[0027] Add appropriate amount of DMF and dichloromethane mixed solvent (30ml) in the reactor, add 3,4-dichloroaniline (10mmol) under stirring, glycolamide (10mmol), copper acetylacetonate catalyst (0.5mmol), N,N '-Dimethylethylenediamine (0.5mmol), NaOH (1mmol), then heated the reactant to 50°C for reaction, reacted for 5 hours, after TLC detection, the reaction was completed, the reaction mixture was poured into saturated saline, stirred rapidly , a large amount of solid precipitated out,
Embodiment 2
[0028] Example 2: Preparation of 6-methyl-1,4-benzoxazin-3(4H)-one
[0029]
[0030] Add appropriate amount of DMF and methylene chloride (30ml) in the reactor, add 4-methyl-1,2-dichlorobenzene (12mmol) under stirring, glycolamide (13mmol), copper acetylacetonate catalyst (0.5mmol), N, N'-dimethylethylenediamine (0.5mmol), KOH (1mmol), then heated the reactant to 60°C for reaction, reacted for 5 hours, and after the reaction was detected by TLC, the reaction mixture was poured into saturated saline , stirred rapidly, a large amount of solids precipitated, filtered with suction and dried to obtain a white solid, yield: 98.4%. 1 H NMR (500MHz, DMSO-d 6 / TMS int ), δ: 2.12 (s, 3H), 4.40 (s, 2H), 6.48-6.73 (m, 3H), 10.82 (s, 1H).
Embodiment 3
[0031] Example 3: Preparation of 6-methoxy-1,4-benzoxazin-3(4H)-one
[0032]
[0033] Add appropriate amount of DMF and dichloromethane (30ml) in the reactor, add 4-methoxy-1,2-dichlorobenzene (12mmol) under stirring, glycolamide (13mmol), copper acetylacetonate catalyst (0.5mmol) , N, N'-dimethylethylenediamine (0.5mmol), NaOH (1mmol), then the reactant was heated to 60°C for reaction, reacted for 5 hours, after the reaction was detected by TLC, the reaction mixture was poured into saturated salt In water, stirred rapidly, a large amount of solids precipitated, suction filtered and dried to obtain a white solid, yield: 99.5%. 1 H-NMR (CDCl 3 -d) δ: 3.75 (s, 3H); 4.55 (s, 2H); 6.40 (d, 1H); 6.50 (dd, 1H); 6.89 (d, 1H); 8.85 (bs, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com