Method for quickly separating and preparing natural dihydroflavonoid compounds by using high-speed counter-current chromatography
A high-speed countercurrent chromatography and dihydroflavonoid technology is applied in the field of preparing dihydroflavonoids by a high-speed countercurrent chromatography continuous cycle separation method, which can solve the problems of long time consumption, single compound structure, and difficulty in one-time separation, etc. high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) The OptiChrome-300PLUS high-speed countercurrent chromatograph produced by Jiangsu Jiangyin Countercurrent Technology Co., Ltd. was used to improve the separation and purification of samples, and the Agilent 1200HPLC high-performance liquid chromatograph produced by Agilent Co., Ltd. of the United States was used for sample purity analysis;
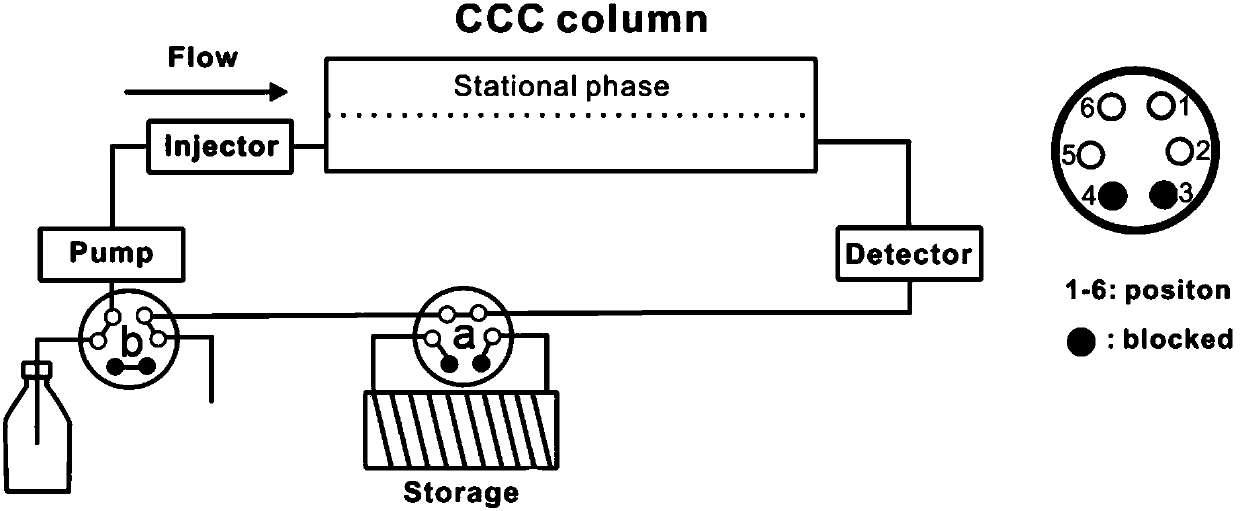
[0034] Set up a high-speed countercurrent chromatograph, connect the six-way valve a to the back of the instrument detector, connect the six-way valve a to the storage ring, and connect the six-way valve b to the front of the constant flow pump, which is responsible for the switching between the two elution modes of normal elution and cycle elution ( Such as figure 1 shown);
[0035] (2) Preparation of extract samples
[0036] Take the dried root of Sophora japonica, crush it, cold soak and extract it with 95% ethanol three times, combine the extracts, concentrate under reduced pressure, and evaporate to dryness;
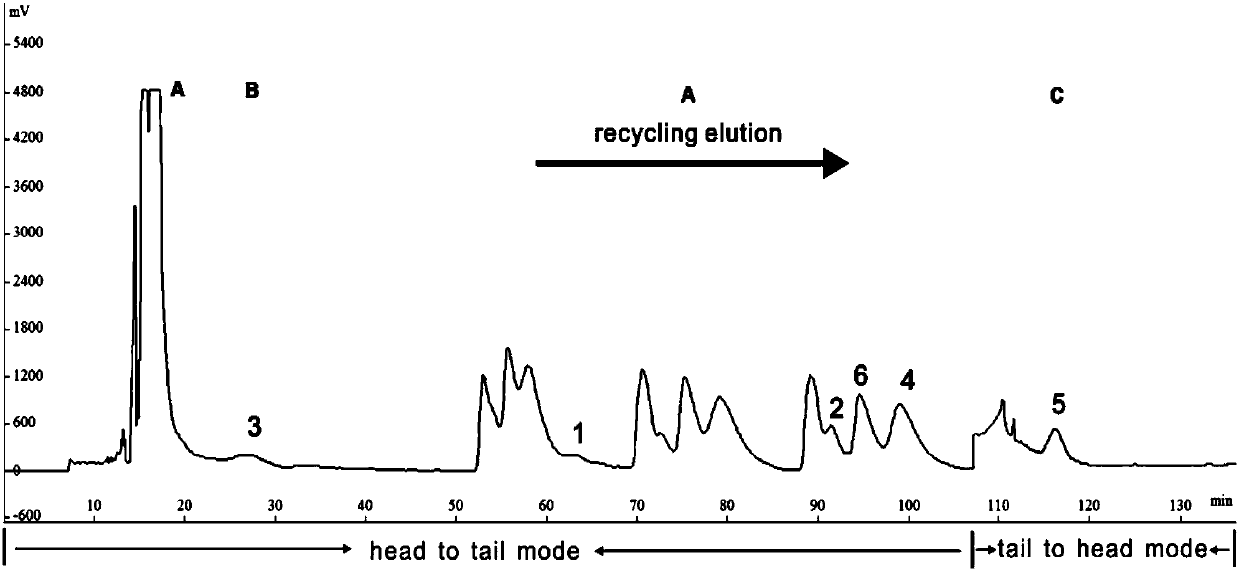
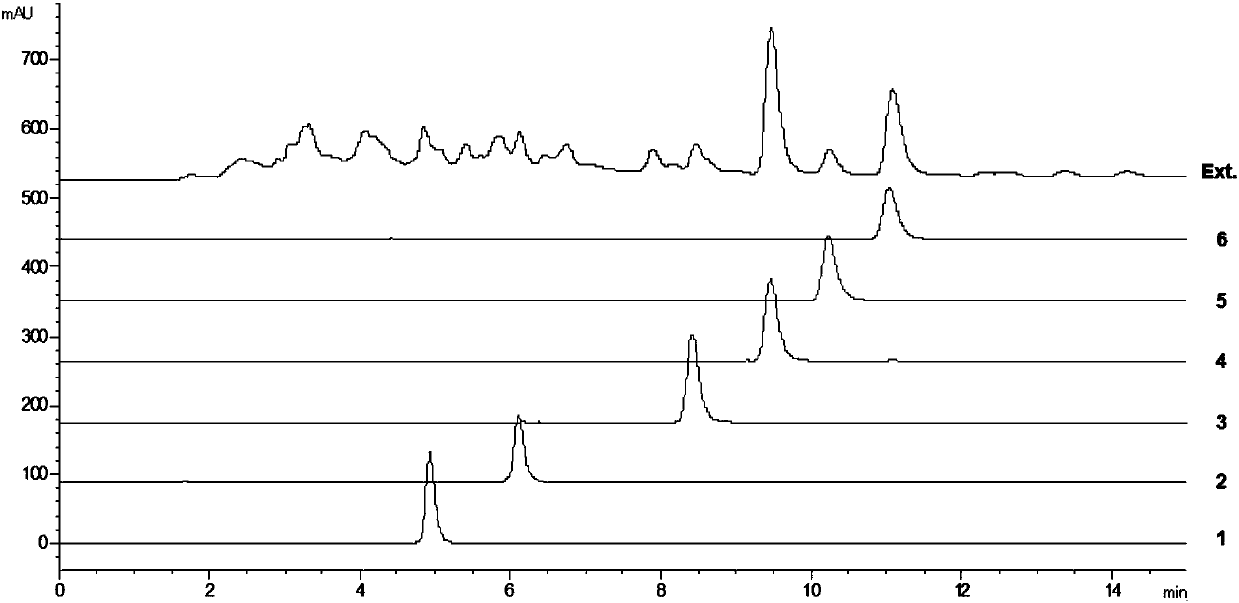
[0037] (3)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com