Application of furanocoumarins in the preparation of antiallergic drugs
A technology of furanocoumarins and compounds, applied in the field of biomedicine, can solve problems such as painful life-threatening, hypersensitivity reactions, and elevated calcium ion concentration in mast cells, and achieve the effect of inhibiting hypersensitivity reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Experimental materials
[0025] Instrument: Automatic microplate reader was purchased from Bio-Rad (California, USA).
[0026] Cell line: KU812 was cultured in IMEM medium with 10% serum, plus 1:100 penicillin-streptomycin. The medium was half-replaced with fresh medium every other day to maintain the cells at 2 × 10 6 density of cells per milliliter.
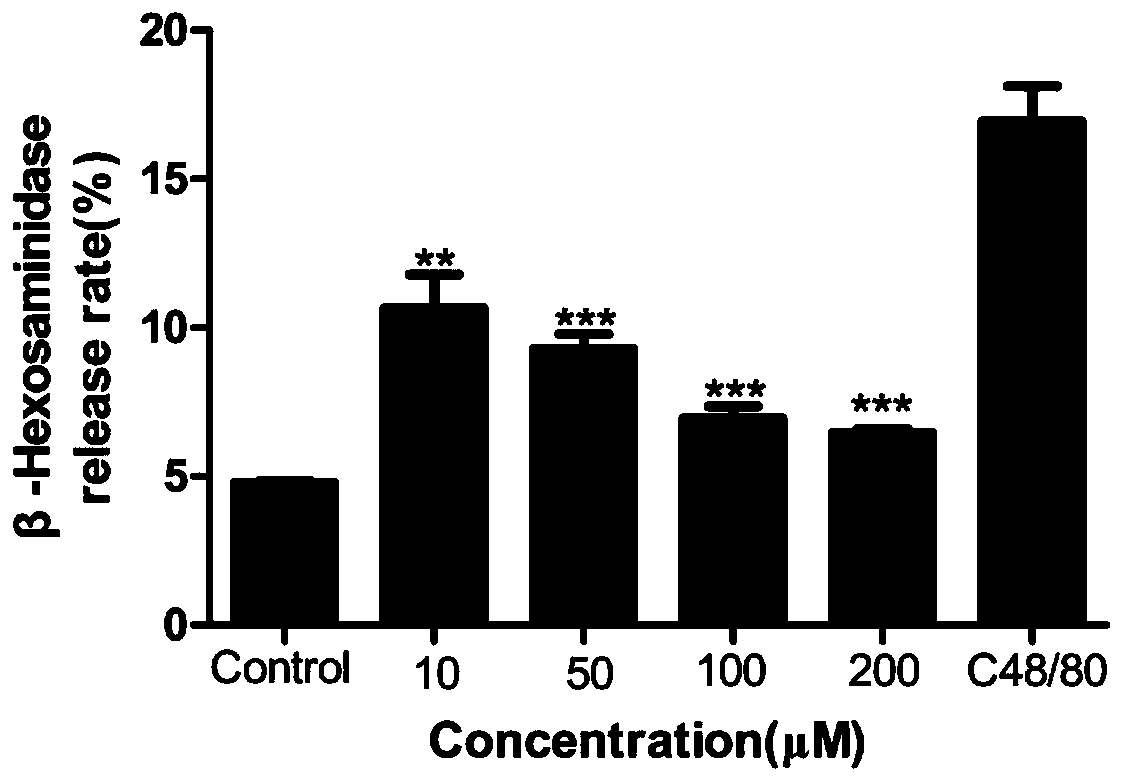
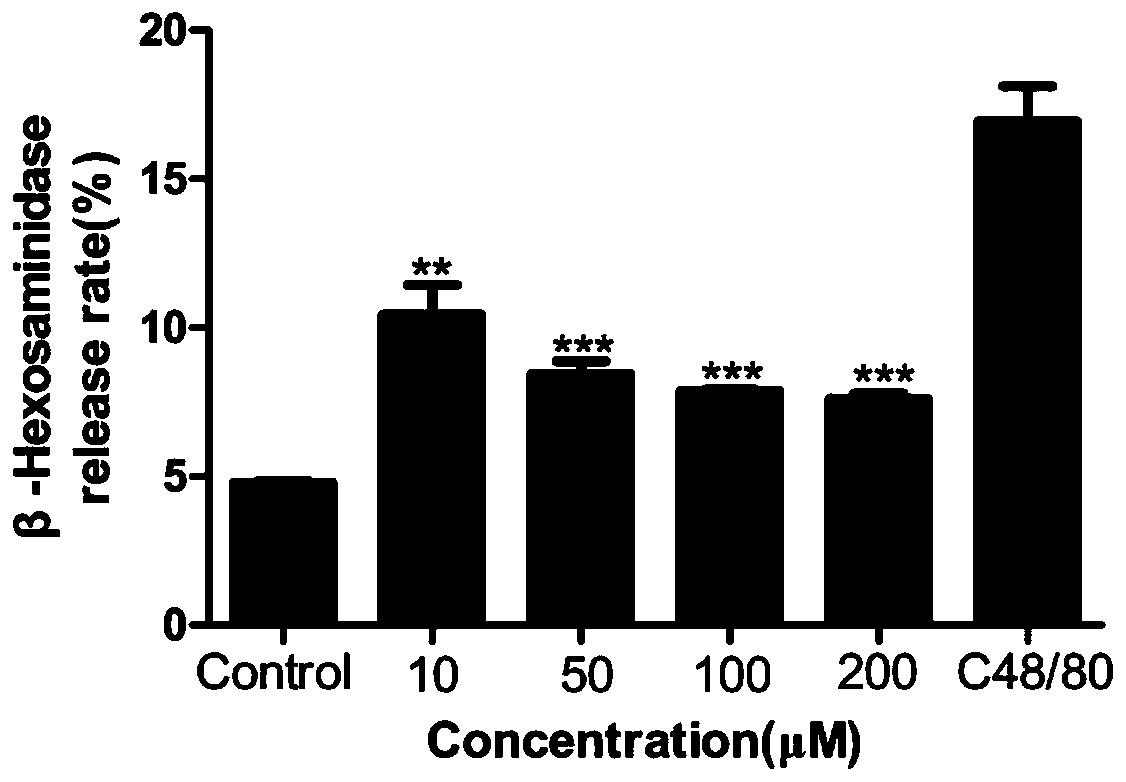
[0027] Main reagent: TM buffer solution (composition (g / L): 6.954 NaCl, 0.353 KCl, 2.383 HEPES, 0.162 KH 2 PO 4 , 0.282 CaCl 2 , 0.143 MgSO 4 , 0.991 glucose, 1 bovine serum albumin. Dimethyl sulfoxide was purchased from Guangzhou Jinhuada Chemical Reagent Co., Ltd., and C48 / 80 was purchased from Sigma-Aldrich (St.Louis, MO, USA). The imperatorin, isoimperatorin, and pectinoids were all purchased from Chengdu Pufeid Biotechnology Co., Ltd. (Chengdu, China). Triton X-100 was purchased from Kehao Bioengineering Company (Xi'an, China), D-glucose Tianjin Kemiou Chemical Reagent Company (Tianjin, China), bovine ser...
Embodiment 2
[0052] 1. Experimental materials
[0053] Instrument: LC-2010AHT (SHIMAZU), self-made column packing machine.
[0054] Cell line: MrgprX2-HEK293 cells were cultured in DMEM medium with 10% fetal bovine serum, plus 1:100 penicillin-streptomycin in 5% CO 2 , in an environment of 37°C. The medium was replaced with fresh medium every other day to keep the cells healthy.
[0055] Main reagents: DMEM medium and fetal bovine serum were purchased from Hyclone (Logan, Utah, USA), and macroporous silica gel was purchased from Qingdao Meigao Group Co., Ltd. (Qingdao, China).
[0056] 2. Experimental method
[0057] 2.1 Preparation of MrgprX2-HEK293 cell membrane stationary phase
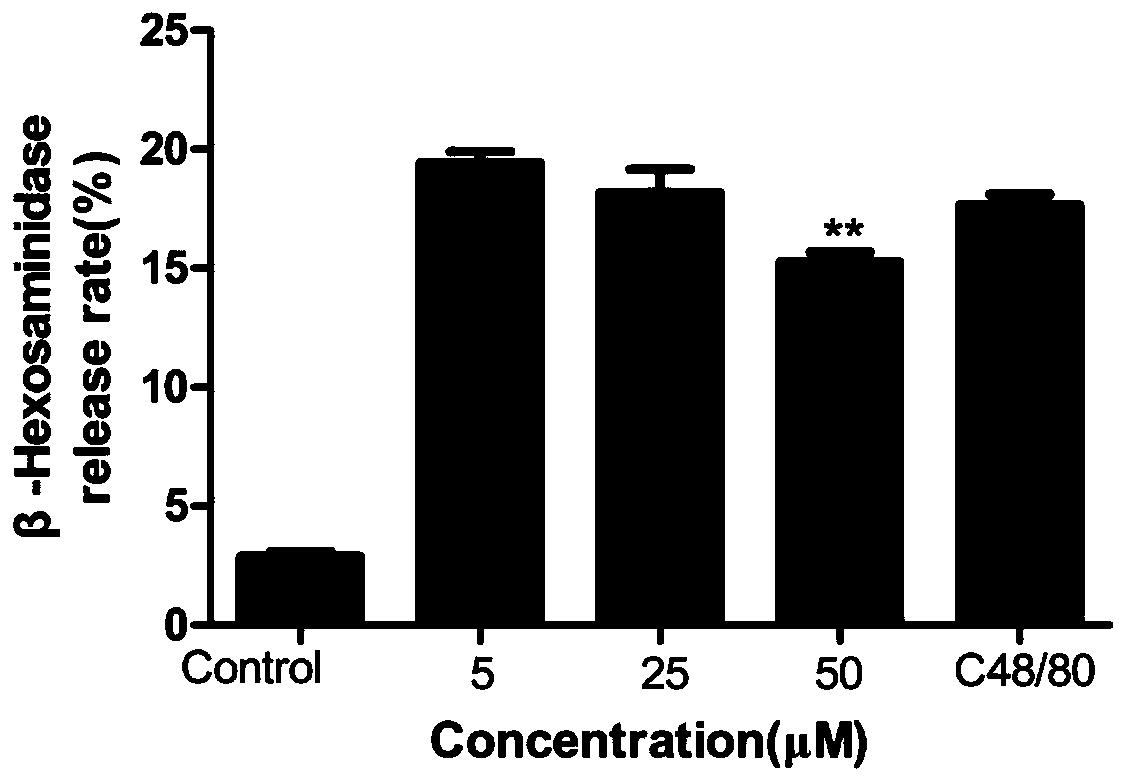
[0058] Take the above-mentioned MrgprX2-HEK293 cells (about 7×10 6 cells) suspension, centrifuge at 1000rpm, 4°C for 5min, discard the supernatant, and take the bottom cells; wash the centrifuged cells twice with 5mL saline, and then add Tris-HCl 5mL to the obtained cells Ultrasound for 30 min in an ice b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com