Medicine composition for treating hemorrhoids, medicinal preparations and application
A technology for pharmaceutical preparations and compositions, applied in the field of pharmaceutical preparations for treating hemorrhoids, can solve the problems of unclear active ingredients and their mechanism of action, large dosage, etc., and achieve the effects of improving side effects and fast curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
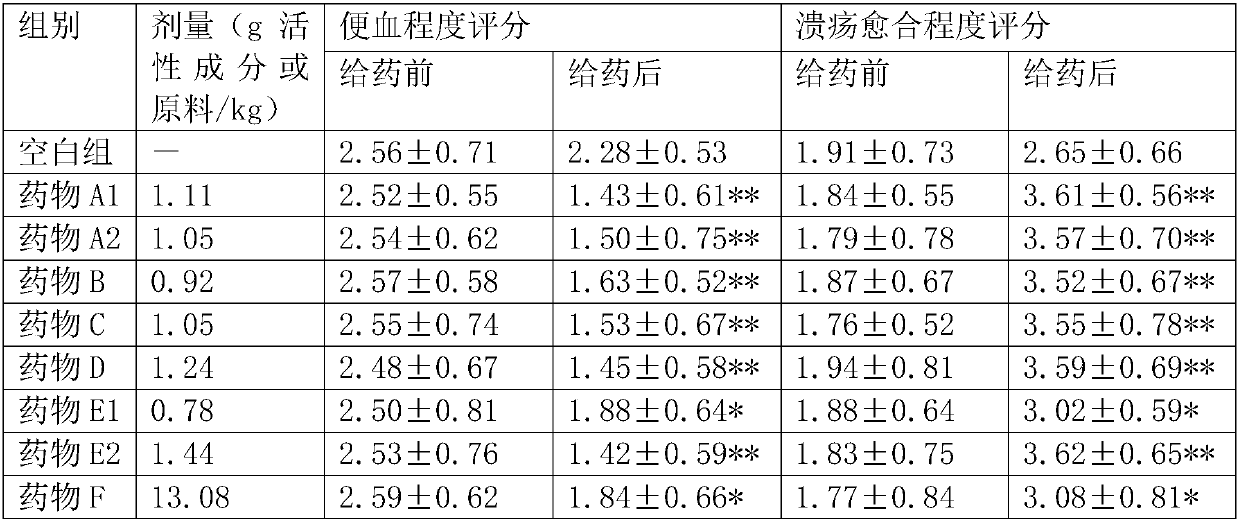
[0029] Embodiment 1: the preparation of A1 suppository
[0030] Prescription: loganin 0.48g, matrine 0.53g, berberine 1.39g, cortex 0.13g, gallic acid 61.38, osthole 2.13g, sweet potato vine total flavonoids 5.05g
[0031] Preparation method: take the formula amount of loganin, matrine, berberine, cortex, gallic acid, osthole, sweet potato vine total flavonoids, mix thoroughly and set aside; take another suppository base to make up the total amount of 637.5g, mix , heated and melted on a water bath, mixed with the above-mentioned thick ointment and medicated powder, and cast into molds to obtain 425 suppositories, each weighing 1.5g; the matrix is polyethylene glycol 4000 and polyethylene glycol 6000 in a mass ratio of :1 mixture. Approximately 0.17g of active ingredient per capsule.
Embodiment 2
[0032] Embodiment 2: the preparation of A2 suppository
[0033] Prescription: loganin 0.58g, matrine 0.60g, berberine 1.25g, cortex 0.14g, gallic acid 57.42g, osthole 2.39g, sweet potato vine total flavonoids 5.59g
[0034] Preparation method: take the formula amount of loganin, matrine, berberine, cortex, gallic acid, osthole, sweet potato vine total flavonoids, mix thoroughly and set aside; take another suppository base to make up the total amount of 637.5g, mix , heated and melted on a water bath, mixed with the above-mentioned thick ointment and medicated powder, and cast into molds to obtain 425 suppositories, each weighing 1.5g; the matrix is polyethylene glycol 4000 and polyethylene glycol 6000 in a mass ratio of : Mixture of 1.2. Each capsule contains about 0.16g of active ingredient.
Embodiment 3
[0035] Embodiment 3: the preparation of B lotion
[0036] Prescription: loganin 0.53g, matrine 0.56g, berberine 1.32g, cortex 0.13g, gallic acid 59.4g, osthole 2.26g, sweet potato vine total flavonoids 5.32g
[0037] Preparation method: Take the formula amount of loganin, matrine, berberine, cortex, gallic acid, osthole, sweet potato vine total flavonoids, add 80% of the total amount of purified water, 0.5% of sodium benzoate, mix well , add water to a total of 1000ml, that is. Each bottle contains 125ml, and the active ingredient is about 0.07g per ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com