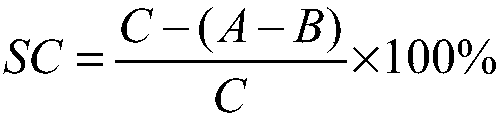Phosphorylated derivative of schizophyllan, and preparation method and application thereof
A technology of schizophyllan and phosphorylation, which is applied in the field of schizophyllan phosphorylated derivatives and their preparation, can solve the problems of expensive derivatization reagents, difficulty in completely dissolving, and limited application, and achieve simple, cheap, and water-soluble derivatization methods. Good sex, good immediate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
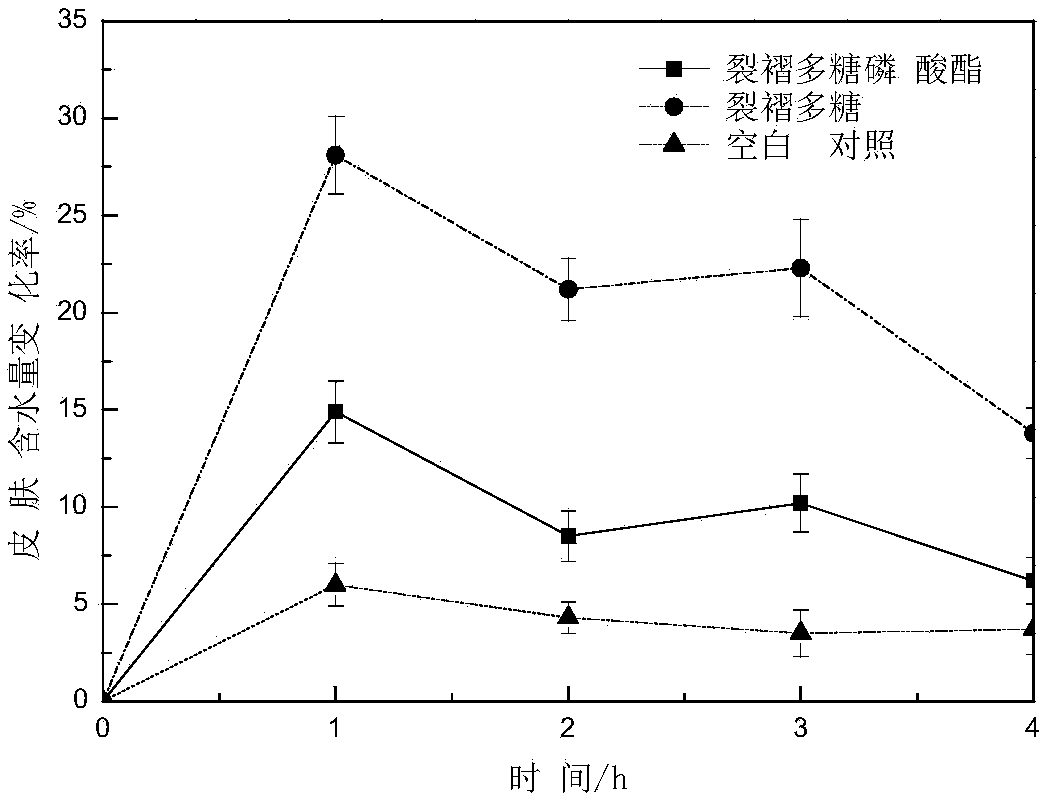
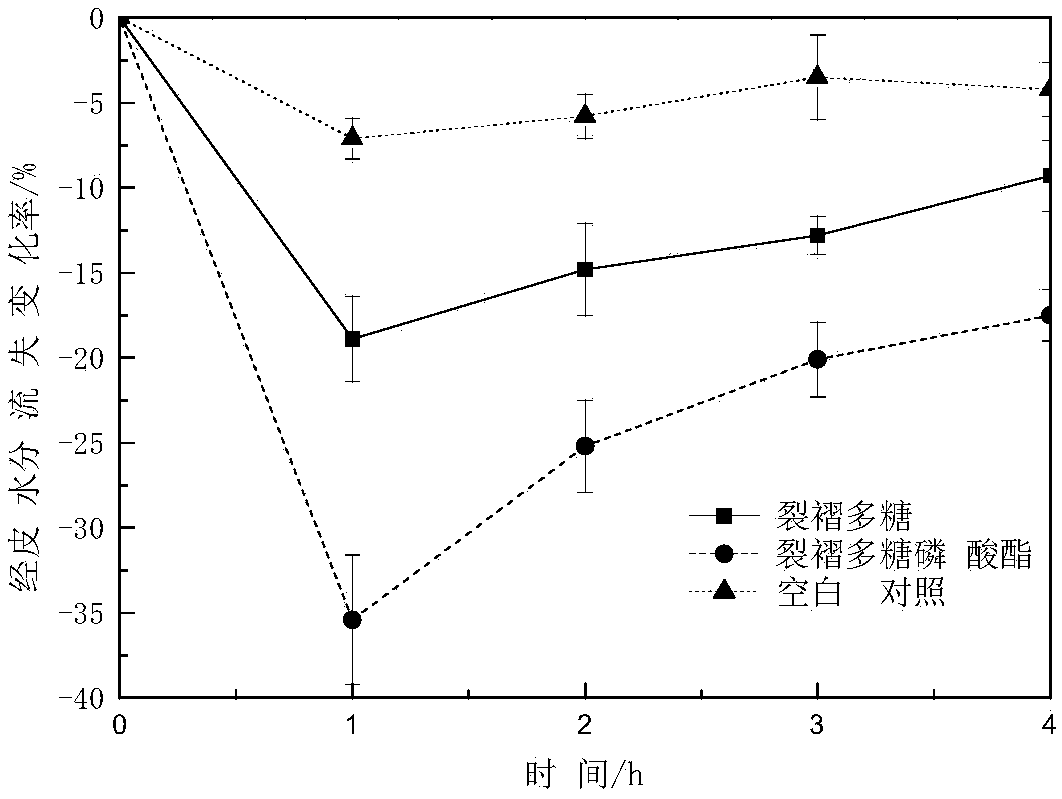
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 Schizophyllan phosphate A
[0029] Dissolve 1 g of Schizophyllan in 20 mL of dimethyl sulfoxide, slowly add 2.5 g of phosphorus pentoxide at a stirring speed of 100 rpm, and keep stirring for 1 h at a temperature of 100 ° C, then cool to room temperature, and Pour the reaction solution into ice water, adjust to neutral with sodium hydroxide solution, add 3 times the amount of ethanol to precipitate, centrifuge, filter, redissolve the precipitate in hot water, dialyze for 1 day, concentrate and freeze-dry to obtain the split fold Polysaccharide Phosphate A.
Embodiment 2
[0030] Preparation of Example 2 Schizophyllan Phosphate B
[0031] Dissolve 1g of Schizophyllan in 100mL of deionized water, slowly add 1g of sodium tripolyphosphate and 1g of sodium trimetaphosphate at a stirring speed of 300rpm, keep stirring for 5 hours at 60°C, and cool to At room temperature, add 3 times the amount of ethanol to the reaction solution to precipitate, centrifuge, filter, redissolve the precipitate in hot water, dialyze for 2 days, concentrate and freeze-dry to obtain Schizophyllan Phosphate B.
Embodiment 3
[0032] Example 3 Preparation of Schizophyllan Phosphate C
[0033] Dissolve 1g of Schizophyllan in 100mL of deionized water, slowly add 1.5g of sodium tripolyphosphate at a stirring speed of 150rpm, and keep stirring for 5 hours at a temperature of 60°C, cool to room temperature, and add 3 Precipitate with twice the amount of ethanol, centrifuge, filter, redissolve the precipitate in hot water, dialyze for 1 day, concentrate and freeze-dry to obtain Schizophyllan Phosphate B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com